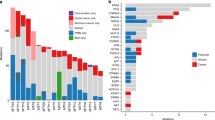
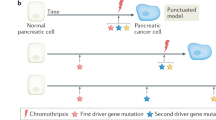
Abstract
Pancreatic intraepithelial neoplasias (PanINs) are the most common precursors of pancreatic cancer, but their small size and inaccessibility in humans make them challenging to study1. Critically, the number, dimensions and connectivity of human PanINs remain largely unknown, precluding important insights into early cancer development. Here, we provide a microanatomical survey of human PanINs by analysing 46 large samples of grossly normal human pancreas with a machine-learning pipeline for quantitative 3D histological reconstruction at single-cell resolution. To elucidate genetic relationships between and within PanINs, we developed a workflow in which 3D modelling guides multi-region microdissection and targeted and whole-exome sequencing. From these samples, we calculated a mean burden of 13 PanINs per cm3 and extrapolated that the normal intact adult pancreas harbours hundreds of PanINs, almost all with oncogenic KRAS hotspot mutations. We found that most PanINs originate as independent clones with distinct somatic mutation profiles. Some spatially continuous PanINs were found to contain multiple KRAS mutations; computational and in situ analyses demonstrated that different KRAS mutations localize to distinct cell subpopulations within these neoplasms, indicating their polyclonal origins. The extensive multifocality and genetic heterogeneity of PanINs raises important questions about mechanisms that drive precancer initiation and confer differential progression risk in the human pancreas. This detailed 3D genomic mapping of molecular alterations in human PanINs provides an empirical foundation for early detection and rational interception of pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
WES data are available via dbGAP under accession number phs003549.v1.p1 as allowed by the Institutional Review Board based on patient consent. Owing to their large file size (TB scale per slab), raw tissue data will be available from the corresponding authors upon request. Source data are provided with this paper.
Code availability
The 3D rendering software used in this Article is available on GitHub at https://github.com/ashleylk/CODA.
References
Hruban, R. H., Goggins, M., Parsons, J. & Kern, S. E. Progression model for pancreatic cancer. Clin. Cancer Res. 6, 2969–2972 (2000).
Siegel, R. L., Miller, K. D., Hannah, F. E. & Jemal, A. Cancer statistics, 2022. CA 72, 7–33 (2022).
Ryan, D. P., Hong, T. S. & Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 371, 1039–1049 (2014).
Takaori, K., Kobashi, Y., Matsusue, S., Matsui, K. & Yamamoto, T. Clinicopathological features of pancreatic intraepithelial neoplasias and their relationship to intraductal papillary-mucinous tumors. J. Hepatobiliary Pancreat. Surg. 10, 125–136 (2003).
Hruban, R. H. et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am. J. Surg. Pathol. 28, 977–987 (2004).
Kanda, M. et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 142, 730–733.e739 (2012).
Hong, S. M. et al. Genome-wide somatic copy number alterations in low-grade PanINs and IPMNs from individuals with a family history of pancreatic cancer. Clin. Cancer Res. 18, 4303–4312 (2012).
Andea, A., Sarkar, F. & Adsay, V. N. Clinicopathological correlates of pancreatic intraepithelial neoplasia: a comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod. Pathol. 16, 996–1006 (2003).
Makohon-Moore, A. P. et al. Precancerous neoplastic cells can move through the pancreatic ductal system. Nature 561, 201–205 (2018).
Kiemen, A. L. et al. CODA: quantitative 3D reconstruction of large tissues at cellular resolution. Nat. Methods 19, 1490–1499 (2022).
Hosoda, W. et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J. Pathol. 242, 16–23 (2017).
Opitz, F. V., Haeberle, L., Daum, A. & Esposito, I. Tumor microenvironment in pancreatic intraepithelial neoplasia. Cancers 13, 6188 (2021).
Hata, T. et al. Genome-wide somatic copy number alterations and mutations in high-grade pancreatic intraepithelial neoplasia. Am. J. Pathol. 188, 1723–1733 (2018).
Chhoda, A., Lu, L., Clerkin, B. M., Risch, H. & Farrell, J. J. Current approaches to pancreatic cancer screening. Am. J. Pathol. 189, 22–35 (2019).
Fischer, C. G. et al. Intraductal papillary mucinous neoplasms arise from multiple independent clones, each with distinct mutations. Gastroenterology 157, 1123–1137.e1122 (2019).
Wu, J. et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 3, 92ra66 (2011).
Felsenstein, M. et al. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut 67, 1652–1662 (2018).
Connor, A. A. et al. Integration of genomic and transcriptional features in pancreatic cancer reveals increased cell cycle progression in metastases. Cancer Cell 35, 267–282.e267 (2019).
Makohon-Moore, A. P. et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 49, 358–366 (2017).
Shi, C. et al. KRAS2 mutations in human pancreatic acinar-ductal metaplastic lesions are limited to those with PanIN: implications for the human pancreatic cancer cell of origin. Mol. Cancer Res. 7, 230–236 (2009).
Qu, C. et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc. Natl Acad. Sci. USA 116, 6308–6312 (2019).
Wang, P. et al. Simultaneous analysis of mutations and methylations in circulating cell-free DNA for hepatocellular carcinoma detection. Sci. Transl. Med. 14, eabp8704 (2022).
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Moore, L. et al. The mutational landscape of human somatic and germline cells. Nature 597, 381–386 (2021).
Aguirre, A. J. et al. High-resolution characterization of the pancreatic adenocarcinoma genome. Proc. Natl Acad. Sci. USA 101, 9067–9072 (2004).
Murphy, S. J. et al. Integrated genomic analysis of pancreatic ductal adenocarcinomas reveals genomic rearrangement events as significant drivers of disease. Cancer Res. 76, 749–761 (2016).
Waddell, N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015).
Zheng, L., Niknafs, N., Wood, L. D., Karchin, R. & Scharpf, R. B. Estimation of cancer cell fractions and clone trees from multi-region sequencing of tumors. Bioinformatics 38, 3677–3683 (2022).
Baker, A.-M. et al. Robust RNA-based in situ mutation detection delineates colorectal cancer subclonal evolution. Nat. Commun. 8, 1998 (2017).
Amano, T. et al. Number of polyps detected is a useful indicator of quality of clinical colonoscopy. Endosc. Int. Open 6, E878–E884 (2018).
Müller, A. D. & Sonnenberg, A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann. Intern. Med. 123, 904–910 (1995).
Rohan, T. E., Henson, D. E., Franco, E. L. & Albores-Saavedra, J. in Cancer Epidemiology and Prevention (eds Schottenfeld, D. & Fraumeni, J. F.) 21–46 (Oxford Univ. Press, 2006).
Williams, A. R., Balasooriya, B. A. & Day, D. W. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 23, 835–842 (1982).
Pollock, P. M. et al. High frequency of BRAF mutations in nevi. Nat. Genet. 33, 19–20 (2003).
Kumar, R., Angelini, S., Snellman, E. & Hemminki, K. BRAF mutations are common somatic events in melanocytic nevi. J. Invest. Dermatol. 122, 342–348 (2004).
Ichii-Nakato, N. et al. High frequency of BRAFV600E mutation in acquired nevi and small congenital nevi, but low frequency of mutation in medium-sized congenital nevi. J. Invest. Dermatol. 126, 2111–2118 (2006).
Cooke, K. R., Spears, G. F. & Skegg, D. C. Frequency of moles in a defined population. J. Epidemiol. Community Health 39, 48–52 (1985).
Schäfer, T., Merkl, J., Klemm, E., Wichmann, H. E. & Ring, J. The epidemiology of nevi and signs of skin aging in the adult general population: results of the KORA-survey 2000. J. Invest. Dermatol. 126, 1490–1496 (2006).
Bryant, K. L., Mancias, J. D., Kimmelman, A. C. & Der, C. J. KRAS: feeding pancreatic cancer proliferation. Trends Biochem. Sci 39, 91–100 (2014).
Chen, Z., Chen, M., Fu, Y. & Zhang, J. The KRAS signaling pathway’s impact on the characteristics of pancreatic cancer cells. Pathol. Res. Pract. 248, 154603 (2023).
Matsuda, Y. et al. The prevalence and clinicopathological characteristics of high-grade pancreatic intraepithelial neoplasia: autopsy study evaluating the entire pancreatic parenchyma. Pancreas 46, 658–664 (2017).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Gabow, H. N. & Myers, E. W. Finding all spanning trees of directed and undirected graphs. SIAM J. Comput. 7, 280–287 (1978).
Niknafs, N., Beleva-Guthrie, V., Naiman, D. Q. & Karchin, R. SubClonal hierarchy inference from somatic mutations: automatic reconstruction of cancer evolutionary trees from multi-region next generation sequencing. PLoS Comput. Biol. 11, e1004416 (2015).
Talevich, E., Shain, A. H., Botton, T. & Bastian, B. C. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput. Biol. 12, e1004873 (2016).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Bergstrom, E. N. et al. SigProfilerMatrixGenerator: a tool for visualizing and exploring patterns of small mutational events. BMC Genomics 20, 685 (2019).
Islam, S. M. A. et al. Uncovering novel mutational signatures by de novo extraction with SigProfilerExtractor. Cell Genomics 2, 100179 (2022).
Olshen, A. B., Venkatraman, E. S., Lucito, R. & Wigler, M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics 5, 557–572 (2004).
Fujikura, K. et al. Multiregion whole-exome sequencing of intraductal papillary mucinous neoplasms reveals frequent somatic KLF4 mutations predominantly in low-grade regions. Gut 70, 928–939 (2021).
Zhao, D. et al. Personalized analysis of minimal residual cancer cells in peritoneal lavage fluid predicts peritoneal dissemination of gastric cancer. J. Hematol. Oncol. 14, 164 (2021).
Zhao, L. et al. Integrated analysis of circulating tumour cells and circulating tumour DNA to detect minimal residual disease in hepatocellular carcinoma. Clin. Transl. Med. 12, e793 (2022).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
McLaren, W. et al. The Ensembl variant effect predictor. Genome Biol. 17, 122 (2016).
Ramos, A. H. et al. Oncotator: cancer variant annotation tool. Hum. Mutat. 36, E2423–E2429 (2015).
Acknowledgements
The authors acknowledge the following sources of support: NIH/NCI P50 CA62924; NIH/NCI T32 CA153952; NIH/NCI U54 CA268083; NIH/NCI U54 CA274371; NIH/NCI U54 CA143868; NIH/NCI U54 CA21073; NIH/NIAMS U54 AR081774; NIH/NCI U01 CA271273; NIH/NIA U01 AG060903; NIH/NIDDK K08 DK107781; NIH/NCI P30 CA00697; Sol Goldman Pancreatic Cancer Research Center; Lustgarten Foundation; Break Through Cancer; Buffone Family Gastrointestinal Cancer Research Fund; Allegheny Health Network-Johns Hopkins Cancer Research Fund; AACR-Bristol-Myers Squibb Midcareer Female Investigator Grant; Rolfe Pancreatic Cancer Foundation; Joseph C. Monastra Foundation; The Gerald O. Mann Charitable Foundation (Harriet and Allan Wulfstat, Trustees); S. Wojcicki and D. Troper; Lustgarten Foundation-AACR Career Development Award for Pancreatic Cancer Research, in honour of Ruth Bader Ginsburg; the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-067 and 2021-1-I2M-018). The authors thank C. Hruban for assistance with illustration. The authors gratefully acknowledge the staff of Oncology Tissue Services at Johns Hopkins, whose expertise in research histology was essential for these studies.
Author information
Authors and Affiliations
Contributions
A.M.B. and A.L.K. contributed equally to the work. L.D.W. and D.W. jointly supervised the work. L.D.W., D.W., R.H.H. and P.-H.W. conceived the project. A.M.B. managed the sectioning and scanning of slabs, tissue microdissection, DNA sequencing and analysis of sequencing results. J.M.B., L.Z., L.J., H.C., Q.S., J.L., R.R., S.G., A.I.D., C.G.F., S.M., C.M., Y.L., J.G., X.-D.L., N.B., L.C.C., F.L. and N.N. supported the scanning and sequencing work. J.P. managed the ISH work and performed analysis. A.L.K. managed the computational 3D reconstruction of slabs, anatomical calculations and visualization of results. M.P.G., A.F., Y.Z., C.A.-P., A.C.J., J.Y., B.K., S.D., E.F., J.Y.H. and P.A.R. supported the 3D reconstruction work. E.K.F., A.Y., N.J.R., E.D.T., R.B.S., T.C.C., Y.J., R.K., R.H.H. and P.-H.W. oversaw various aspects of the work. A.M.B., L.D.W., A.L.K. and D.W. created the first draft of the manuscript and figures. J.P., L.D.W., A.L.K. and A.M.B. prepared the revised manuscript and figures, which all authors edited and approved.
Corresponding authors
Ethics declarations
Competing interests
A pending patent application ‘Computational Techniques For Three-Dimensional Reconstruction and Multi-labeling of Serially Sectioned Tissue’ was filed on 24 June 2022 by A.L.K., R.H.H., P.-H.W., D.W. and L.D.W. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Tim Coorens, Beatrice Knudsen, Marina Pasca di Magliano and Hiroki Ueda for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Validation of CODA segmentation.
A. H&E cell detection true positives (TP), false positives (FP), false negatives (FN), precision, and recall compared to manual annotations by two individuals. B. Confusion matrix detailing performance of semantic segmentation algorithm in labelling pancreatic microanatomy in H&E stained tissue images.
Extended Data Fig. 2 CODA-rendered 3D models for tissue slabs analyzed.
Blue represents normal pancreatic ducts; spatially separate PanINs are indicated with distinct colors.
Extended Data Fig. 3 Quantified features from CODA-rendered 3D models of human pancreatic tissue slabs.
A. PanIN burden stratified by disease type. PanINs per cm3 of analyzed tissue for each slab, not statistically significant. Mean with standard deviations plotted. n = 38 independent samples. B. PanIN burden stratified by sex. PanINs per cm3 of analyzed tissue for each slab, not statistically significant. Mean with standard deviations plotted. n = 38 independent samples. C. PanIN burden stratified by location of harvested tissue, not statistically significant. Mean with standard deviations plotted. n = 38 independent samples. D. PanIN burden stratified by age, not statistically significant as determined by simple linear regression. E. Percent ductal system affected by PanIN by disease type. Percentage of neoplastic ductal cells was calculated for each slab, not statistically significant by two-tailed Mann-Whitney test. + = mean. n = 38 independent samples. Whiskers denote min-max values while box shows Q1–Q3 with median denoted by a line. F. Number of cells comprising PanINs for each slab by disease type, not statistically significant by two-tailed Mann-Whitney test. n = 38 independent samples. Whiskers show min-max values while box shows Q1–Q3 with median value denoted by a line. G. Correlation of percent PanIN cells (x-axis) to percent normal ductal cells (y-axis). Each point represents a tissue slab. H. Correlation of percent PanIN cells (x-axis) to percent cells in ECM (y-axis). I. Correlation of percent PanIN cells (x-axis) to percent acinar cells (y-axis). J. Correlation of percent PanIN cells (x-axis) to percent smooth muscle cells (y-axis). Correlation of percent PanIN cells (x-axis) to percent islet cells (y-axis). L. Correlation of percent PanIN cells (x-axis) to percent fat cells (y-axis). G-L. r2 and p-values calculated using the correlation coefficient, and grey shading represents the 95-percent confidence interval.
Extended Data Fig. 4 KRAS oncogenic hotspot mutations in PanINs assessed by multiregional targeted NGS of eight tissue slabs and mutation burden by lesion grade from WES.
A. Missense mutations occur primarily at G12 and at lower frequencies in Q61. B. At the G12 locus, four different amino acid substitutions were found, with p.G12D being the most common. C. Number of mutations per region stratified by low grade, high grade, and PDAC. Mean with standard deviation plotted. p < 0.0001 when comparing mutations in low grade and PDAC samples; determined by Kruskal-Wallis test, corrected for multiple comparisons with Dunn’s test. 48 low grade PanINs, 4 high grade PanINs, and 9 PDACs from 38 independent samples were assessed.
Extended Data Fig. 5 Copy number aberrations in PDAC and PanINs analyzed by WES.
A. Chromosome arm-level copy number changes summarized as Z-scores, shown in the precursor and matched tumor samples from each individual. Z-score levels falling within the observed range of the composite panel of normals are depicted in white (copy neutral). For values above or below the normal range, the distance to the maximum or minimum value within the normal range are reported. B-I. Chromosome arm level copy number aberrations in representative sample, slab 92. The blue ribbon marks the range of z-scores observed in the panel of normals.
Extended Data Fig. 6 Slabs 151, 155, 117: 3D model and NGS results.
A-C: slab 151. A. CODA-rendered 3D model of slab 151, with multiple spatially distinct PanINs (color key at right). B Mutation chart with targeted sequencing results for slab 151. Each row represents a distinct somatic mutation. Columns represent PanINs, colored as in A; bars represent vertical regions within a PanIN. Bar color corresponds to key on right. C. Heatmap of VAFs from Mutation Capsule KRAS deep sequencing of for slab 151. Rows represent different KRAS oncogenic hotspot mutations. Columns represent PanINs, with colors corresponding to A. D-G: slab 155. D. CODA-rendered 3D model of grossly normal pancreatic tissue in slab 155, with multiple spatially distinct PanINs (color key at right). E. Mutation chart with targeted sequencing results for slab 155. Each row represents a distinct somatic mutation. Columns represent PanINs, colored as in D; bars represent vertical regions within a PanIN. Bar color corresponds to key on right. F. Chow-Ruskey plot of WES results for slab 155. Shapes represent groups of mutations, with overlaps indicating shared somatic mutations. Numbers refer to the number of mutations in each group. G. Heatmap of VAFs from Mutation Capsule KRAS deep sequencing of slab 155. Rows represent different KRAS oncogenic hotspot mutations. Columns represent PanINs, with colors corresponding to D. H-J: slab 117. H. CODA-rendered 3D model of grossly normal pancreatic tissue in slab 117, with multiple spatially distinct PanINs (color key at right). I. Mutation chart with targeted sequencing results for slab 117. Each row represents a distinct somatic mutation. Columns represent PanINs, with colors as in H; bars represent vertical regions within a PanIN. Bar color corresponds to key on right. J. Heatmap of VAFs from Mutation Capsule KRAS deep sequencing. Rows represent different KRAS oncogenic hotspot mutations. Columns represent PanINs, with colors as in H.
Extended Data Fig. 7 Slabs 114 and 116: 3D model and NGS results.
A-D: slab 114. A. CODA-rendered 3D model of grossly normal pancreatic tissue in slab 114, with multiple spatially distinct PanINs (color key at right). B. Mutation chart with targeted sequencing results for slab 114. Each row represents a distinct somatic mutation. Columns represent PanINs; bars represent vertical regions within a PanIN. Bar color corresponds to key on right. C. Chow-Ruskey plot summarizing WES results for slab 114. Each shape represents a group of mutations. Colors correspond to PanINs in A, except matched PDAC sample sequenced from archival clinical block (grey). Shapes represent groups of mutations, with overlaps indicating shared somatic mutations. Numbers refer to the number of mutations in each group. D. Heatmap of VAFs from Mutation Capsule KRAS deep sequencing of slab 114. Rows represent different KRAS oncogenic hotspot mutations. Columns represent PanINs, colored as in A, except matched PDAC sample sequenced from archival clinical block (grey). E-H: slab 116. E. CODA-rendered 3D model of grossly normal pancreatic tissue in slab 116, with multiple spatially distinct PanINs (color key at right). F. Mutation chart with targeted sequencing results for slab 116. Each row represents a distinct somatic mutation. Columns represent PanINs; bars represent vertical regions within a PanIN. Bar color corresponds to key on right. G. Chow-Ruskey plot summarizing WES results for slab 116. Each shape represents a group of mutations. Colors correspond to PanINs in E except matched PDAC sample sequenced from archival clinical block (grey). Shapes represent groups of mutations, with overlaps indicating shared somatic mutations. Numbers refer to the number of mutations in each group. H. Heatmap of VAFs from Mutation Capsule KRAS deep sequencing of slab 116. Rows represent different KRAS oncogenic hotspot mutations. Columns represent PanINs, except matched PDAC sample sequenced from archival clinical block (grey).
Extended Data Fig. 8 Normalized quantification of KRAS G12 point mutation-specific RNA ISH for each PanIN, shown with CODA-rendered 3D models in corresponding colors.
Tissue sections from a single region in each following slab were analyzed for point mutations in KRAS G12. LG and HG areas were quantified and depicted separately when present. Corresponding VAFs from targeted NGS of each PanIN region shown below each column, with most common variant highlighted in grey. Lavender arrows mark PanIN regions where KRAS Q61H mutations were found in NGS. A. Slab 114. B. Slab 151. C. Slab 155. D. Slab 104. E. Slab 98. For the following slabs containing PanINs with multiple KRAS mutations by NGS, tissue sections were analyzed as above from two regions. F. Slab 116. G. Slab 117.
Extended Data Fig. 9 Representative images of mutant and total KRAS RNA ISH staining in histologically normal ductal epithelial cells adjacent to PanIN.
A. KRAS G12R mutant RNA ISH probe staining (red puncta) in a PanIN and adjacent normal ductal cells, with consecutive H&E-stained tissue section. B. Universal KRAS reference ISH probe staining of same PanIN and adjacent normal ductal cells, with corresponding H&E image. Scale bars = 50 µm.
Supplementary information
Supplementary Table 1
Summary of samples and clinical characteristics.1.0 Patient samples and analyses performed for entire cohort. 1.1 Clinical and pathological data for normal pancreas samples. 1.2 PanIN samples and sequencing analyses performed for slabs analysed by combined 3D genomic mapping.
Supplementary Table 2
Microanatomical quantifications of CODA-rendered 3D models. 2.0 Microanatomical quantifications for cases analysed by 3D modelling only. 2.1 Microanatomical quantifications for cases analysed by 3D genomic mapping. 2.2 Quantitative features of individual PanINs. 2.3 Comparison of PanIN burden in 3D modelling and genomic mapping cohorts.
Supplementary Table 3
Data quality and somatic mutations identified by targeted sequencing. 3.0 Genomic regions targeted by NGS. 3.1 Summary of quality metrics for targeted sequencing data. 3.2 Summary of slide numbers processed and lesions obtained from each case. 3.3–3.10 Somatic mutations identified by targeted sequencing of slabs 92, 98, 104, 114, 116, 117, 151 and 155, respectively.
Supplementary Table 4
Somatic mutations identified by deep sequencing of KRAS hotspots. 4.0 Mutations identified by deep sequencing of KRAS hotspots.
Supplementary Table 5
Data quality and somatic mutations identified by WES. 5.0 Summary of quality metrics for WES data. 5.1–5.8 Somatic mutations identified by WES of slabs 92, 98, 104, 114, 116, 117, 151 and 155, respectively.
Supplementary Video 1
Example 3D bounding box for one contiguous PanIN. Video extracted from the registered 5x magnification image stack. Image stacks were manually viewed using FIJI ImageJ for accuracy of PanIN identification.
Supplementary Video 2
Image sequence of registered serial H&E sections from slab 92. Spatially separate PanIN were recognized using CODA and annotated on the registered sections for aid in separate microdissection of distinct lesions.
Supplementary Video 3
3D rendering of spatially separate PanIN in slab 92. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green.
Supplementary Video 4
3D rendering of spatially separate PanIN in slab 98. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green.
Supplementary Video 5
3D rendering of spatially separate PanIN in slab 104. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green.
Supplementary Video 6
3D rendering of spatially separate PanIN in slab 114. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green.
Supplementary Video 7
3D rendering of spatially separate PanIN in slab 116. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green.
Supplementary Video 8
3D rendering of spatially separate PanIN in slab 117. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green.
Supplementary Video 9
3D rendering of spatially separate PanIN in slab 151. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green
Supplementary Video 10
3D rendering of spatially separate PanIN in slab 155. Ten largest PanINs coloured distinctly. Additional smaller PanINs coloured in olive green.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Braxton, A.M., Kiemen, A.L., Grahn, M.P. et al. 3D genomic mapping reveals multifocality of human pancreatic precancers. Nature 629, 679–687 (2024). https://doi.org/10.1038/s41586-024-07359-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07359-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.