Abstract
Checkpoint inhibitor-based immunotherapy is increasingly used in the treatment of gynecologic cancers, and most often targets the PD-1/PD-L1 axis. Pathologists should be familiar with the biomarkers required to determine candidacy for these treatments based on existing FDA approvals, including mismatch repair protein immunohistochemistry, microsatellite instability testing, tumor mutation burden testing, and PD-L1 immunohistochemistry. This review summarizes the rationale behind these treatments and their associated biomarkers and delivers guidance on how to utilize and readout these tests. It also introduces additional biomarkers which may provide information regarding immunotherapeutic vulnerability in the future such as neoantigen load; POLE mutation status; and immunohistochemical expression of immunosuppressive checkpoints like LAG-3, TIM-3, TIGIT, and VISTA; immune-activating checkpoints such as CD27, CD40, CD134, and CD137; enzymes such as IDO-1 and adenosine-related compounds; and MHC class I.
Similar content being viewed by others
Introduction
The interactions between the immune system and malignancy have long been appreciated and, over the course of the last two decades, have successfully been harnessed for therapeutic purposes in cancers, including some gynecologic tumors1,2,3,4. Gynecologic cancers include a diverse array of malignancies of ovarian, uterine, cervical, and vulvar origin and show variable vulnerability to both established and emerging immunotherapies5,6,7. Their responses to some of these therapies appear to be tied in part to genetic and protein expression characteristics including mismatch repair status, tumor mutational burden, and checkpoint ligand expression.
Immunotherapy is a broad term that encompasses a variety of techniques including immune checkpoint inhibitors and stimulators, therapeutic cancer vaccines, and adoptive cellular transfer. While some of these treatments are now commonly enlisted in the clinical setting, others remain largely restricted to the research sphere. We herein discuss the current state of immune checkpoint-based therapy across gynecologic cancer types, with an emphasis on the pathologist’s role in reading out these biomarkers and triaging tissue specimens to optimize the selection of gynecologic oncology patients for immunotherapeutic access.
The therapeutic rationale for targeting immune checkpoints
Manipulation of immune checkpoints represents the most successful and widely enlisted strategy of immunotherapy across tumor types. These drugs capitalize on the inveterate “checks and balances” built into the human immune system: immune checkpoints are co-signaling pathways that can either enhance or suppress the immune response when T cells engage antigen-presenting cells—including tumor cells that have co-opted this system for their own benefit. In a normally functioning immune response, the equilibrium between immune-activating and immunosuppressive checkpoints ensures that reactions to immunogenic stimuli do not propagate inexorably, protecting the host against autoimmunity and promoting self-tolerance. Malignancies, however, may hijack these pathways to avoid recognition and attack by the host’s immune system.
Although checkpoint-based immunotherapy can target either immune-activating or immunosuppressive molecules, immunosuppressive checkpoint blockers have seen far more clinical success thus far. The most clinically relevant immunosuppressive checkpoint receptors are programed cell death (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), each of which tamps down the immune response through interaction with its ligands (PD-L1/PD-L2 and B7-1/B7-2, respectively)3,8. Therapies targeting these pathways include antibodies directed at both the receptors and their ligands. Anti-CTLA-4 drugs were developed first and continue to play a role in the care of melanoma patients, however, they are associated with significant immune-related toxicities9. Although there is some evidence that they can synergize with PARP inhibition in BRCA1-deficient ovarian cancers10, overall anti-CTLA-4 therapies have failed to gain significant traction in the treatment of gynecologic tumors. Antibodies targeting PD-1 and its ligand PD-L1 have proven more broadly effective and less toxic, showing tolerability and durable responses among patients with a variety of malignancies including melanoma, non-small cell lung carcinoma, and urothelial carcinomas11,12,13. More recently, these drugs have shown to benefit a subset of gynecologic cancer patients, including some women with endometrial cancer14,15,16,17; cervical squamous cell carcinoma and adenocarcinoma18,19; vulvar squamous cell carcinoma20; ovarian carcinoma21,22; gestational trophoblastic tumors23; and even occasional uterine sarcomas24,25,26,27. (Table 1) Among endometrial cancers, mismatch repair-deficient/microsatellite unstable cancers are particularly vulnerable to anti-PD-1 therapy28, although mismatch repair-intact/microsatellite stable advanced and recurrent cancers can also benefit17, particularly in the context of polymerase epsilon (POLE) mutations29,30.
The pathologist’s role: current FDA-approved biomarkers for checkpoint inhibitor candidacy in gynecologic carcinoma
Pathologists play a critical role in identifying which gynecologic cancers will be candidates for checkpoint inhibition. At present, there are Food & Drug Administration (FDA)-approved pathways for treatment with the PD-1 inhibitor pembrolizumab based on the presence of mismatch repair-deficiency/high-level microsatellite instability15,31 or high tumor mutational burden32,33 in any advanced solid tumor—including gynecologic cancers. The anti-PD-1 drug dostarlimab is also approved for advanced mismatch repair-deficient endometrial cancers using a specific companion diagnostic assay (Ventana MMR RxDx)34. In addition, recurrent and metastatic cervical carcinomas are FDA-approved pembrolizumab candidates if they have positive PD-L1 expression using the companion diagnostic 22C3 pharmDX assay35. Details regarding the rationale behind and application of these biomarkers are detailed below.
Mismatch repair deficiency/microsatellite instability
In 2017 Le et al. published data in Science showing that over half of progressive mismatch repair-deficient solid tumors treated pembrolizumab demonstrate appreciable responses, including over 20% with complete response15. This led to the ground-breaking FDA approval of pembrolizumab in advanced solid tumors demonstrating mismatch repair deficiency or high-level microsatellite instability, representing the agency’s first tumor type and site-agnostic recommendation tied to a molecular feature15,31 (Tables 1–2). These impressive immunotherapeutic responses make biologic sense: when malignancies are unable to successfully repair DNA mismatches they rapidly accumulate mutations (often on the order of 50–100 per megabase) which leads to enhanced neoantigen production36. This high neoantigen load, in turn, triggers increased immune recognition that drives adaptive resistance through the expression of PD-L1 and other immunosuppressive molecules36,37,38,39,40. Although these adaptations help the tumor thrive despite a cytotoxic T cell-enriched milieu, they also render the tumor vulnerable to immune attack when checkpoint inhibitors such as pembrolizumab are applied15.
While this FDA approval significantly expanded the pool of cancer patients who could receive immunotherapy, confusion quickly ensued regarding which test was optimal for selecting potential responders as the approval was not tied to a companion assay or even a specific kind of test41. Many used “mismatch repair deficiency” and “microsatellite instability” interchangeably, however, these terms refer to results produced from entirely different assays which have variable performance characteristics in different tumor types. Mismatch repair deficiency is conventionally defined by immunohistochemical loss of expression of one or more of the four main mismatch repair proteins (MLH1, PMS2, MSH2, and MSH6). (Fig. 1) Microsatellite instability, in contrast, is a PCR or NGS-based test that evaluates repetitive regions of the genome (microsatellites) which are vulnerable to expansion and contraction in the context of an improperly functioning mismatch repair system. Moreover, some labs can perform DNA sequencing which can directly identify aberrations in mismatch repair gene sequences. Not surprisingly, pathologists and oncologists have been left wondering which assay is most appropriate.
This endometrioid carcinoma (A) demonstrates robust tumor-associated lymphocytes (B), which are attributable to the elevated mutation burden and high neoantigen load associated with underlying mismatch repair deficiency. Although MLH1 (C), MSH2 (not pictured), and MSH6 (not pictured) expression was intact, PMS2 (D) shows total loss of expression within tumor cell nuclei with preserved positive internal control staining in background stroma and associated lymphocytes. Genetic testing in this patient revealed a germline PMS2 mutation. Based on the presence of mismatch repair deficiency, this tumor would qualify for anti-PD-1 checkpoint inhibitor therapy.
To ameliorate this uncertainty, in 2018 the College of American Pathologists (CAP) convened an expert panel to review data on mismatch repair-associated biomarkers and immunotherapeutic response in solid tumors42. Because there is a paucity of direct data relating mismatch repair biomarker results to immunotherapy response, the panel relied largely on data derived from the Lynch syndrome literature, assessing biomarker results in comparison to a gold-standard of germline-confirmed mismatch repair mutations. While the final iterations of these guidelines are under development, preliminary recommendations were released for open comment from February 19-March 13 202043.
The CAP panel’s drafted recommendations stated that mismatch repair immunohistochemistry is an acceptable assay for checkpoint inhibitor access across tumor types—including gynecologic cancers. Moreover, immunohistochemistry is preferred over microsatellite instability testing in non-colorectal carcinomas as commercially available microsatellite instability assays are optimized for colorectal carcinoma, and have been less well-validated outside of this tumor type. In addition, microsatellite instability testing has decreased sensitivity for MSH6 mutations, which are enriched in endometrial cancers when compared to colorectal cancers44,45. Importantly, only complete loss of expression in the presence of adequate internal control staining constitutes true deficiency using immunohistochemistry. Although subclonal loss patterns can be observed46, their significance with regard to immunotherapeutic vulnerability is unknown. It is also unclear whether retesting on recurrence specimens is warranted, as mismatch repair status changes over time have not been well studied and cases with mismatch repair status evolution were not included in checkpoint inhibitor trials. Finally, the panel cautioned that while DNA sequencing represents a promising methodology for the detection of mismatch repair gene defects, there are limited data regarding the performance characteristics of this approach in this specific context. The final iteration of the CAP’s Guidelines for mismatch repair resting for immunotherapy access are expected by late 2021, and should be consulted for final updates.
It is worth noting that while the 2017 FDA approval for pembrolizumab in mismatch repair-deficient/microsatellite instability-high tumors did not require a specific assay31, more recently the Ventana MMR RxDx assay has been linked to the PD-1 inhibitor dostarlimab as a companion diagnostic in endometrial carcinoma34. It remains to be seen whether future FDA approvals will include such companion diagnostic requirements, and pathologists should be aware that this may impact their ability to determine candidacy for certain drugs based on mismatch repair status without confirming assay interchangeability.
At present, mismatch repair-related biomarkers are typically applied based on the treating clinician’s request as checkpoint inhibitors are not currently considered front-line in any gynecologic carcinoma types. At many institutions, including our own, immunohistochemical staining for mismatch repair proteins is already performed reflexively on all endometrial carcinomas and some ovarian carcinomas (specifically those with clear cell and endometrioid histologies) as part of universal Lynch syndrome screening47,48, therefore in these tumor types mismatch repair immunohistochemistry need not be repeated for immunotherapeutic access. In other gynecologic cancers where this testing is not performed reflexively, mismatch repair immunostaining may be requested for patients who have progressed on standard of care chemotherapy and may provide a novel treatment option for occasional patients49,50,51,52.
PD-L1 expression
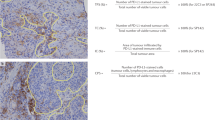
In 2017, data from the KEYNOTE-158 study revealed that 14% of PD-L1-positive cervical carcinomas that had failed prior therapy respond to pembrolizumab, including just under 3% with complete responses18,35. This led to the 2018 FDA approval of pembrolizumab to treat advanced cervical cancers that express PD-L1 on immunohistochemistry (22C3 pharmDX kit, Agilent)35 (Tables 1–2). Because all responders expressed PD-L1 with a Combined Positive Score (CPS) ≥1, PD-L1 immunohistochemistry was approved as a biomarker for pembrolizumab access at this positivity threshold. The CPS PD-L1 scoring system was originally developed for upper gastrointestinal tumors and accounts for both tumor cell and tumor-associated immune cell staining using the following equation: [(total number of PD-L1-positive tumor cells, lymphocytes, and macrophages) / (total number of viable tumor cells)] x 10053,54. (Fig. 2) The threshold lower limit (CPS = 1) requires only an average of one PD-L1 positive tumor cell, lymphocyte, or macrophage per 100 tumor cells. Although many cervical carcinomas are much more flagrantly PD-L1 positive, it is not clear that increased expression correlates with improved response to therapy. Details regarding how to properly readout a PD-L1 CPS are provided elsewhere53,54,55,56.
This cervical squamous cell carcinoma (A) demonstrates strong diffuse membranous PD-L1 expression (B) on tumor cells as well as cytoplasmic and membranous staining in tumor-associated macrophages and lymphocytes. Given that the stain is positive on essentially every tumor cell, this carcinoma qualifies for the maximum combined positive score (CPS) of 100. Because a minimum score of only 1 is required for FDA-approved access to anti-PD-1 immunotherapy, this tumor readily clears the threshold for treatment candidacy.
Importantly, the FDA approval for pembrolizumab in cervical cancer is specifically tied to the 22C3 pharmDX Kit on the Dako Autostainer. Although PD-L1 expression has been shown to vary somewhat across different antibody clones and staining instruments, there is also evidence that some assays can produce comparable results at clinically significant thresholds57,58,59. It may therefore be reasonable to consider validating alternate PD-L1 biomarker assays with the same or different anti-PD-L1 primary antibody clones to facilitate testing in settings where the FDA-approved assay is unavailable. However, it is key that the validation design is appropriate for predictive immunohistochemical biomarker assays and that relevant guidelines for laboratory-developed tests are followed, including both technical and clinical validation60,61
At the time of writing, cervical carcinoma is the only gynecologic cancer in which PD-L1 immunostaining is enlisted for checkpoint inhibitor access based on PD-L1 CPS status, however, that may change in the future. Vulvar squamous cell carcinomas, in particular, represent attractive candidates for PD-L1 biomarkers as these tumors have considerable biologic overlap with cervical squamous cell carcinomas and can show impressive responses to pembrolizumab20. Given the rapidly expanding role of PD-L1 immunohistochemistry as a biomarker for checkpoint inhibitor access in other organ systems, it would be not be surprising if a new FDA approval tied to this biomarker emerged. Fortunately, the principles discussed for PD-L1 in cervical carcinoma are likely to be transferable to other gynecologic sites, provided the CPS remains the preferred scoring methodology as it has in most other organ systems.
Tumor mutational burden
In 2020 the KEYNOTE-158 study revealed that 29% of patients with solid tumors showing high-level tumor mutational burden (defined as ≥10 mutations per megabase and using the FoundationOne CDx assay) showed durable responses to monotherapy with pembrolizumab, including 4% with complete responses32. Their data revealed that while high tumor mutation burden often overlapped with high-level microsatellite instability and/or PD-L1 immunohistochemical expression, this correspondence was not absolute. This molecular signature therefore became an additional FDA-approved avenue for anti-PD-1 access among solid tumors in 202033. (Tables 1–2).
Although high tumor mutational burdens are seen in roughly 10% of gynecologic malignancies62,63, tumor mutational burden testing for immunotherapeutic access has not yet penetrated routine practice for gynecologic cancer care in most settings. This is in part because endometrial cancers and cervical cancers are often candidates for checkpoint inhibition through other FDA-approved avenues and the other most common gynecologic cancer type, high-grade ovarian serous carcinoma, has alternate therapeutic options such as PARP inhibitors. Nonetheless, pathologists should be aware that oncologists may request that formalin-fixed tumor tissue be sent for this testing, and should be prepared to select tumor-containing blocks as appropriate.
Future directions
Although FDA-approved assays like mismatch repair testing and PD-L1 immunohistochemistry identify many gynecologic cancer patients as candidates for checkpoint inhibitor therapy, the majority still fail to demonstrate durable treatment responses. Moreover, these FDA-approved biomarkers do not necessarily capture all responders. The potential reasons for this are myriad due to the incredible intricacy of the tumor-host immune interaction. Additional biomarkers that further characterize this interaction may therefore be of value in optimizing the patient selection for immunotherapy. Emerging biomarkers that could help identify strong candidates for checkpoint inhibition include neoantigen load and POLE mutation status as well as immunohistochemistry for other immunosuppressive checkpoints, immune-activating checkpoints, immunosuppressive enzymes, and molecules necessary for adaptive immune recognition.
Neoantigen load
Cancer-specific neoantigens are accrued due to genetic alterations that lead to novel sequences of amino acids which have not been previously recognized by the immune system, and DNA sequencing is to infer the overall burden of these neoantigens using bioinformatics algorithms64. Neoantigen loads have been shown to vary dramatically across gynecologic tumors64 but are particularly high among mismatch repair-deficient endometrial cancers36 and BRCA-mutated high-grade serous ovarian cancers65. Moreover, higher neoantigen loads are generally correlated with higher tumor mutational burdens, higher levels of immune infiltration, and better overall survival64. However, while neoantigen load often runs parallel to tumor mutational burden, the two are not interchangeable, and neoantigen load may theoretically identify tumors with lower mutational burdens in which mutations are particularly immunogenic. While it is not yet well established that neoantigen load significantly contributes to the identification of responders when compared to related technologies like tumor mutational burden testing, preliminary data suggests that it could have independent value in predicting clinical benefit to immunotherapy66, and this test may have significance as a biomarker in the future.
Polymerase epsilon (POLE) status
Mutations in POLE—the gene encoding the DNA polymerase epsilon catalytic subunit—also impart vulnerability to checkpoint inhibitor-based immunotherapy. Hotspot mutations in POLE are fairly common in endometrial carcinoma with up to 10% of all endometrial cancers falling into the molecular subgroup defined by these mutations67. These DNA proofreading defects lead to an impressive accumulation of mutations that exceeds even what is seen in the context of mismatch repair deficiency, with POLE-mutated tumors demonstrating as many as 500 mutations per megabase67. Multiple trials investigating PD-1/PD-L1 inhibitors in POLE-mutated gynecologic cancers are underway (clinicaltrials.gov: NCT04463771; NCT04774419; NCT04267939; NCT03012581), and initial data suggests that the anti-PD-1 drug nivolumab has promise in mismatch repair-intact endometrial cancers with pathogenic POLE mutations29,30. POLE mutations can also be found in 3% of cervical carcinomas and 1–2% of ovarian carcinomas and may eventually represent a useful future immunotherapeutic biomarker in these tumor types as well68.
Targetable checkpoints
The PD-1/PD-L1 axis is one of many immunosuppressive checkpoint pathways that malignancies can enlist to evade the host immune response. Other immunosuppressive checkpoint molecules that have been identified in gynecologic carcinomas include lymphocyte-activation gene (LAG-3)39,69,70,71, T cell immunoglobulin and mucin domain (TIM-3)40,72,73,74,75, T cell immunoreceptor with Ig and ITIM domains (TIGIT)76,77,78, and V-domain immunoglobulin (Ig)-containing suppressor of T-cell activation (VISTA)79,80,81. (Table 3) Expression of some of these molecules and of their ligands is particularly prominent in the setting of mismatch repair deficiency, and they are often co-expressed with PD-L139,40,71,72. This suggests that for many gynecologic malignancies, anti-PD-1/anti-PD-L1 monotherapy may be inadequate, and that combination approaches targeting multiple immune checkpoint pathways may find more success. Multiple trials investigating drugs targeting these pathways are underway in gynecologic cancers (clinicaltrials.gov: NCT03250832; NCT03489343; NCT03708328; NCT02817633; NCT04475523; NCT04693234; NCT04693234; NCT04570839) and may inform our approach to checkpoint inhibitor therapy and biomarker testing in the future.
There may also be a role for biomarkers and drugs targeting immunostimulatory checkpoints. Examples of immunostimulatory checkpoints that have shown early promise in the investigative setting include CD27, CD40, CD134 (OX40), CD137 (4-1BB); these molecules are in the immunoglobulin (Ig) and tumor necrosis factor (TNF) families and interact with their ligands to enhance T cell survival, proliferation, and differentiation82,83,84 (Table 3). Clinical trials investigating the role of immunostimulatory checkpoint agonism are ongoing for a variety of solid tumors—including gynecologic cancers (clinicaltrials.gov: NCT04406623; NCT01644968; NCT02410512; NCT02335918), and may help to identify successful combination immunotherapy approaches in these tumors.
Targetable enzymes
Immunosuppressive enzymes may also confound attempts to enhance the anti-tumoral response using checkpoint inhibitors. Enzymes that have shown promise as immunotherapeutic targets include indoleamine dioxygenase 2, 3 and adenosine-related compounds. Indoleamine dioxygenase 2, 3-1 (IDO1) is an immunoregulatory enzyme induced by interferon-gamma85,86,87,88,89,90,91,92. Its immunosuppressive function is tied to its metabolization of tryptophan, which lymphocytes require for survival, and the generation of a toxic metabolite known as kynurenine. It plays a key role in blocking T-cell expansion and enhancing the immunosuppressive properties of some dendritic cells and is critical for promoting fetal tolerance and in curbing autoimmunity87,89,90,93,94,95. IDO1 expression has been demonstrated in endometrial, cervical, and ovarian cancers, with particularly high expression in the settings of high-level PD-L1 expression and mismatch repair deficiency38,96,97,98,99,100. (Fig. 3) Drugs targeting IDO are in clinical trials in a variety of carcinoma types and are of particular interest in combination with anti-PD-1/PD-L1 check point inhibitors (clinicaltrials.gov: NCT03459222; NCT04106414). The related molecule indoleamine dioxygenase 2, 3-2 (IDO2) may also have a role in immunotherapy but has been less well-studied101,102.
Enzymes related to adenosine production have also shown promise in immunotherapy. Adenosine is a nucleoside derivative of adenosine triphosphate (ATP) which accumulates in response to cellular stress and breakdown. It has anti-inflammatory function through the generation of its secondary messenger, cyclic adenosine monophosphate (cAMP), which upregulates transforming growth factor-beta (TGF-ß) and immune-inhibitory checkpoints such as PD-1103. Immunotherapeutic targets of interest in the adenosine pathway include the adenosine A 2 A receptor (A2AR, ADORA2A) and CD73 (NT5E)103,104,105,106. Clinical trials of immunotherapies directed at these targets are underway in a variety of solid tumor types, including gynecologic cancers. (NCT03719326; NCT03629756; NCT03719326; NCT04148937, NCT03255252; NCT03267589).
Other mechanisms of immune evasion
Finally, some gynecologic cancers appear capable of evading the host’s adaptive immune system altogether through the loss of major histocompatibility (MHC) class I. MHC class I is expressed on the surface of all nucleated human cells and functions like a flag pole for the display of intracellular antigens, interacting with T cells to promote a cytotoxic response when foreign antigens are present107,108,109. The presence of MHC class I on a tumor cell’s surface is thus critical for enlistment of a CD8+ T cell adaptive anti-tumoral immune response, and a decrease or loss of MHC class I expression represents a putative mechanism of immune evasion in some cancers110,111,112,113. Among gynecologic malignancies, nearly half of endometrial carcinomas and more than a third of HPV-associated cervical squamous carcinomas have been shown to have complete or partial loss of MHC class I, potentially limiting the efficacy of adaptive immunotherapeutic approaches in these cancers irrespective of other biomarker statuses114,115. (Fig. 4) Interestingly, loss of MHC class I should render tumors more vulnerable to recognition by the innate immune system, particularly NK cells, due to the “non-human” nature of MHC class I-deficient cells116. Although innate immune responses are thought to be inadequate for the control for well-established tumors, MHC class I deficiency may suggest increased vulnerability to emerging immunotherapies that enhance innate immune effectors such as NK cells116,117. In addition, recent evidence suggests that MHC class I loss may confer increased susceptibility to chimeric antigen receptor T cell-based immunotherapy118. Such approaches, however, are currently limited to the investigative sphere.
Although all normal nucleated human cells express MHC Class I, loss of expression can be seen in malignancies and represents a mechanism of adaptive immune evasion. Loss may be total, as seen in the endometrial carcinoma depicted in (A, B), or partial, as in the endometrial carcinoma illustrated in (C, D). Notably, both these cancers were mismatch repair-deficient. Evidence from other tumor types suggests that MHC class I loss may confer resistance to checkpoint inhibitor-based immunotherapy, and studies are underway to determine the significance of this biomarker in the gynecologic tract.
In summary, although tumor responses to checkpoint inhibitors can be influenced by far more than currently FDA-approved biomarkers like mismatch repair status, tumor mutational burden, and PD-L1 expression, it remains to be seen whether any additional biomarkers become relevant for routine clinical practice. One could certainly imagine a diagnostic setting in which additional molecular assays or a panel of immunohistochemical stains could be used to optimize immunotherapy candidate selection and curate tumor-specific combination immunotherapeutic approaches, however, clinical trials are needed to critically assess the value of such an approach. In the short term, it is worthwhile for pathologists to understand the rationale behind current FDA-approved checkpoint inhibitor therapies, know how to apply and readout their associated biomarkers, and appreciate that many new biomarkers may be on the horizon.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3, 991–998 (2002).
Reiss, K. A., Forde, P. M. & Brahmer, J. R. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy 6, 459–475 (2014).
Taube, J. M. et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 20, 5064–5074 (2014).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl J. Med. 366, 2443–2454 (2012).
Bourla, A. B. & Zamarin, D. Immunotherapy: new strategies for the treatment of gynecologic malignancies. Oncology 30, 59–66,69 (2016).
Murali, R., Grisham, R. N. & Soslow, R. A. The roles of pathology in targeted therapy of women with gynecologic cancers. Gyn. Oncol. 148, 213–221 (2018).
Gadducci, A. & Guerrieri, M. E. Immune checkpoint inhibitors in gynecological cancers: update of literature and perspectives of clinical research. Anticancer Res. 37, 5955–5965 (2017).
Chambers, C. A., Kuhns, M. S., Egen, J. G. & Allison, J. P. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19, 565–594 (2001).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Higuchi, T. et al. CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol. Res. 3, 1257–1268 (2015).
Robert, C. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384, 1109–1117 (2014).
Garon, E. B. et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl J. Med. 372, 2018–2028 (2015).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl J. Med. 376, 1015–1026 (2017).
Le, D. T. et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Le, D. T. et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 28, 409–413 (2017).
Ott, P. A. et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1–positive endometrial cancer: results from the KEYNOTE-028 study. J. Clin. Oncol. 35, 2535–2541 (2017).
Makker, V. et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 20, 711–718 (2019).
Frenel, J. S. et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J. Clin. Oncol. 35, 4035–4041 (2017).
Chung, H. C. et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 37, 1470–1478 (2019).
Shields, L. B. E. & Gordinier, M. E. Pembrolizumab in recurrent squamous cell carcinoma of the vulva: case report and review of the literature. Gynecol. Obstet. Invest. 84, 94–98 (2019).
Bellone, S. et al. Exceptional response to pembrolizumab in a metastatic, chemotherapy/radiation-resistant ovarian cancer patient harboring a PD-L1-genetic rearrangement. Clin. Cancer Res. 24, 3282–3291 (2018).
Hamanishi, J. et al. Safety and antitumor activity of Anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol. 33, 4015–4022 (2015).
You, B. et al. Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: cohort A of the TROPHIMMUN Phase II trial. J. Clin. Oncol. 38, 3129–3137 (2020).
Ben-Ami, E. et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: results of a phase 2 study. Cancer 123, 3285–3290 (2017).
D’Angelo, S. P. et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 19, 416–426 (2018).
Tawbi, H. A. et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 18, 1493–1501 (2017).
Groisberg, R. et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J. Immunother Cancer 5, 100 (2017).
Zhao, P., Li, L., Jiang, X. & Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 12, 54 (2019).
Rousseau, B. J. et al. 526O high activity of nivolumab in patients with pathogenic exonucleasic domain POLE (edPOLE) mutated Mismatch Repair proficient (MMRp) advanced tumours. Ann. Oncol. 31, S463 (2020).
Santin, A. D. et al. Regression of chemotherapy-resistant polymerase epsilon (POLE) ultra-mutated and MSH6 hyper-mutated endometrial tumors with nivolumab. Clin. Cancer Res. 22, 5682–5687 (2016).
Marcus, L., Lemery, S. J., Keegan, P. & Pazdur, R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 25, 3753–3758 (2019).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
FDA approves pembrolizumab for adults and children with TMB-H solid tumors. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors. Accessed 31 Dec 2020.
FDA Approves VENTANA MMR RxDx as Companion Diagnostic for Dostarlimab in Endometrial Cancer. https://www.onclive.com/view/fda-approves-ventana-mmr-rxdx-as-companion-diagnostic-for-dostarlimab-in-endometrial-cancer. Accessed 11 May 2021.
FDA approves pembrolizumab for advanced cervical cancer with disease progression during or after chemotherapy. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm610572.htm. Accessed 7 May 2021.
Howitt, B. E. et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol. 1, 1319–1323 (2015).
Sloan, E. A., Ring, K. L., Willis, B. C., Modesitt, S. C. & Mills, A. M. PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including lynch syndrome-associated and MLH1 promoter hypermethylated tumors. Am. J. Surg. Pathol. 41, 326–333 (2017).
Chinn, Z., Stoler, M. H. & Mills, A. M. PD-L1 and IDO expression in cervical and vulvar invasive and intraepithelial squamous neoplasias: implications for combination immunotherapy. Histopathology 74, 256–268 (2019).
Friedman, L. A., Ring, K. L. & Mills, A. M. LAG-3 and GAL-3 in endometrial carcinoma: emerging candidates for immunotherapy. Int. J. Gyn. Pathol. 39, 203–212 (2020).
Moore, M., Ring, K. L. & Mills, A. M. TIM-3 in endometrial carcinomas: an immunotherapeutic target expressed by mismatch repair-deficient and intact cancers. Mod. Pathol. 32, 1168–1179 (2019).
Ledford, H. Promising cancer drug hits snags. Nature 544, 13–14 (2018).
MMR and MSI testing in patients being considered for checkpoint inhibitor therapy. https://www.cap.org/protocols-and-guidelines/cap-guidelines/upcoming-cap-guidelines/mmr-and-msi-testing-in-patients-being-considered-for-checkpoint-inhibitor-therapy. Accessed 7 May 2021.
CAP Opens Comment Period for MMR/MSI testing advancing care for patients with cancer. https://www.cap.org/news/2020/cap-opens-comment-period-for-mmr-msi-testing-advancing-care-for-patients-with-cancer. Accessed 7 May 2021.
Wu, X. et al. Minimal microsatellite shift in microsatellite instability high endometrial cancer: a significant pitfall in diagnostic interpretation. Mod. Pathol. 32, 650–658 (2019).
de Leeuw, W. J. et al. Prediction of a mismatch repair gene defect by microsatellite instability and immunohistochemical analysis in endometrial tumours from HNPCC patients. J. Pathol. 192, 328–335 (2000).
Watkins, J. C., Nucci, M. R., Ritterhouse, L. L., Howitt, B. E. & Sholl, L. M. Unusual mismatch repair immunohistochemical patterns in endometrial carcinoma. Am. J. Surg. Pathol. 40, 909–916 (2016).
Mills, A. M. et al. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am. J. Surg. Pathol. 38, 1501–1509 (2014).
Mills, A. M. & Longacre, T. A. Lynch syndrome screening in the gynecologic tract: current state of the art. Am. J. Surg. Pathol. 40, e35–e44 (2016).
Leskela, S. et al. Mismatch repair deficiency in ovarian carcinoma: frequency, causes, and consequences. Am. J. Surg. Pathol. 44, 649–656 (2020).
Schmoeckel, E. et al. Comprehensive analysis of PD-L1 expression, HER2 amplification, ALK/EML4 fusion, and mismatch repair deficiency as putative predictive and prognostic factors in ovarian carcinoma. Virchows Arch. 474, 599–608 (2019).
Bonneville R., et al. Landscape of microsatellite instability across 39 cancer types. JCO Precision Oncol. 1–15 (2017).
Jensen, K. C. et al. Microsatellite instability and mismatch repair protein defects in ovarian epithelial neoplasms in patients 50 years of age and younger. Am. J. Surg. Pathol. 32, 1029–1037 (2008).
Kulangara, K. et al. Development of the combined positive score (CPS) for the evaluation of PD-L1 in solid tumors with the immunohistochemistry assay PD-L1 IHC 22C3 pharmDx. J. Clin. Oncol. 35, e14589–e14589 (2017).
Kulangara, K. et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Path. Lab. Med. 143, 330–337 (2019).
Paxton A. Scoring gastric, GEJ cancers for PD-L1 expression. CAP Today (2018).
Mills, A. M. PD-L1 interpretation in cervical carcinomas: proceedings of the ISGyP companion society session at the 2020 USCAP annual meeting. Int. J. Gyn. Pathol. 40, 1–4 (2021).
Rimm, D. L. et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non–small cell lung cancer. JAMA Oncol. 3, 1051–1058 (2017).
Marchetti, A. et al. Multicenter comparison of 22C3 pharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J. Thorac. Oncol.12, 1654–1663 (2017).
Fujimoto, D. et al. Comparison of PD-L1 assays in non-small cell lung cancer: 22C3 pharmdx and SP263. Anticancer Res. 38, 6891–6895 (2018).
Cheung, C. C. et al. Fit-for-purpose PD-L1 biomarker testing for patient selection in immuno-oncology: guidelines for clinical laboratories from the Canadian Association of Pathologists-Association Canadienne Des Pathologistes (CAP-ACP). Appl. Immunohistochem Mol. Morphol. 27, 699–714 (2019).
Fitzgibbons, P. L. et al. Principles of analytic validation of immunohistochemical assays: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch. Pathol. Lab. Med. 138, 1432–1443 (2014).
Huang, R. S. P. et al. A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod. Pathol. 34, 252–263 (2021).
Shao, C. et al. Prevalence of high tumor mutational burden and association with survival in patients with less common solid tumors. JAMA Netw. Open 3, e2025109 (2020).
Zhu, Y. et al. Characterization of neoantigen load subgroups in gynecologic and breast cancers. Front. Bioeng Biotechnol. 8, 702 (2020).
Strickland, K. C. et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget 7, 13587–13598 (2016).
Kim, K. et al. Predicting clinical benefit of immunotherapy by antigenic or functional mutations affecting tumour immunogenicity. Nat. Commun. 11, 951 (2020).
Talhouk, A. et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 113, 299–310 (2015).
AACR Project GENIE Consortium. AACR project GENIE: powering precision medicine through an international consortium. Cancer Disco 7, 818–831 (2017).
Goldberg, M. V. & Drake, C. G. LAG-3 in cancer immunotherapy. Curr. Top Microbiol. Immunol. 344, 269–278 (2011).
He, Y. et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J. Thorac. Oncol. 12, 814–823 (2017).
Whitehair, R., Peres, L. C. & Mills, A. M. Expression of the immune checkpoints LAG-3 and PD-L1 in high-grade serous ovarian carcinoma: relationship to tumor-associated lymphocytes and germline BRCA status. Int. J. Gyn. Pathol. 39, 558–566 (2020).
Curley, J. et al. Looking past PD-L1: expression of immune checkpoint TIM-3 and its ligand galectin-9 in cervical and vulvar squamous neoplasia. Mod. Pathol. 33, 1182–1192 (2020).
Du, W., et al. TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int. J. Mol. Sci. 18, 645 (2017).
Guo, Z. et al. Combined TIM-3 blockade and CD137 activation affords the long-term protection in a murine model of ovarian cancer. J. Transl. Med. 11, 215 (2013).
Cao, Y. et al. Tim-3 expression in cervical cancer promotes tumor metastasis. PloS ONE 8, e53834 (2013).
Chen, F., Xu, Y., Chen, Y. & Shan, S. TIGIT enhances CD4+ regulatory T‐cell response and mediates immune suppression in a murine ovarian cancer model. Cancer Med. 9, 3584–3591 (2020).
Johnston, R. J. et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26, 923–937 (2014).
Smazynski, J. et al. The immune suppressive factors CD155 and PD-L1 show contrasting expression patterns and immune correlates in ovarian and other cancers. Gynecol. Oncol. 158, 167–177 (2020).
Lines, J. L., Sempere, L. F., Broughton, T., Wang, L. & Noelle, R. VISTA Is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol. Res. 2, 510–517 (2014).
ElTanbouly, M. A., Croteau, W., Noelle, R. J., Lines, J. L. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Sem. Immunol. 42 (2019).
Abadier, M. LeyK. P-selectin glycoprotein ligand-1 in T cells. Curr. Opin. Hematol. 24, 265–273 (2017).
Buchan, S. L., Rogel, A. & Al-Shamkhani, A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 131, 39–48 (2018).
Bulliard, Y. et al. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol. Cell Biol. 92, 475–480 (2014).
Scarlett, U. K. et al. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 69, 7329–7337 (2009).
Yan, Y. et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 185, 5953–5961 (2010).
Leung, B. S., Stout, L. E., Shaskan, E. G. & Thompson, R. M. Differential induction of indoleamine-2,3-dioxygenase (IDO) by interferon-gamma in human gynecologic cancer cells. Cancer Lett. 66, 77–81 (1992).
Fallarino, F. et al. IDO mediates TLR9-driven protection from experimental autoimmune diabetes. J. Immunol. 183, 6303–6312 (2009).
Iversen, T. Z., Andersen, M. H. & Svane, I. M. The targeting of indoleamine 2,3 dioxygenase -mediated immune escape in cancer. Basic Clin. Pharm. Toxicol. 116, 19–24 (2015).
Munn, D. H. & Mellor, A. L. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34, 137–143 (2013).
Munn, D. H. & Mellor, A. L. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 37, 193–207 (2016).
Platten, M., von Knebel, Doeberitz, Oezen, I., Wick, W. & Ochs, K. Cancer immunotherapy by targeting IDO1/TDO and their downstream effectors. Front. Immunol. 5, 673 (2015).
Vacchelli, E. et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 3, e957994 (2014).
Munn, D. H. et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 (1998).
Suzuki, S., Toné, S., Takikawa, O., Kubo, I. & Minatogawa, Y. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem. J. 355, 425–429 (2001).
Szántó, S. et al. Inhibition of indoleamine 2,3-dioxygenase-mediated tryptophan catabolism accelerates collagen-induced arthritis in mice. Arthritis Res. Ther. 9, R50 (2007).
Mills, A. M. et al. Targetable immune regulatory molecule expression in high-grade serous ovarian carcinomas in African American women: a study of PD-L1 and IDO in 112 cases from the African American Cancer Epidemiology Study (AACES). Int. J. Gyn. Pathol. 38, 157–170 (2019).
Mills, A. M. et al. Indoleamine 2,3-dioxygenase in endometrial cancer: a targetable mechanism of immune resistance in mismatch repair-deficient and intact endometrial carcinomas. Mod. Pathol. 31, 1282-1290 (2018)
Inaba, T. et al. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol. Oncol. 115, 185–192 (2009).
Inaba, T. et al. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol. Oncol. 117, 423–428 (2010).
de Jong, R. A. et al. Prognostic role of indoleamine 2,3-dioxygenase in endometrial carcinoma. Gynecol. Oncol. 126, 474–480 (2012).
Mondanelli, G. et al. Current challenges for IDO2 as target in cancer immunotherapy. Front. Immunol. 12 (2021).
Metz, R. et al. IDO2 is critical for IDO1-mediated T-cell regulation and exerts a non-redundant function in inflammation. Int. Immunol. 26, 357–367 (2014).
Leone, R. D. & Emens, L. A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer 6, 57 (2018).
Halpin-Veszeleiova, K. & Hatfield, S. M. Oxygenation and A2AR blockade to eliminate hypoxia/HIF-1α-adenosinergic immunosuppressive axis and improve cancer immunotherapy. Curr. Opin. Pharm. 53, 84–90 (2020).
Allard, D., Allard, B., Gaudreau, P.-O., Chrobak, P. & Stagg, J. CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy 8, 145–163 (2016).
Chen, S. et al. CD73 expression on effector T cells sustained by TGF-β facilitates tumor resistance to anti-4-1BB/CD137 therapy. Nat. Commun. 10, 150 (2019).
Neefjes, J., Jongsma, M. L., Paul, P. & Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 11, 823–836 (2011).
Kloor, M. et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 65, 6418–6424 (2005).
Šmahel M. PD-1/PD-L1 blockade therapy for tumors with downregulated MHC class I expression. Intl. J. Mol. Sci. 18, 1331 (2017).
Ugurel, S. et al. MHC class-I downregulation in PD-1/PD-L1 inhibitor refractory Merkel cell carcinoma and its potential reversal by histone deacetylase inhibition: a case series. Cancer Immunol. Immunother. 68, 983–990 (2019).
Yoo, S. H. et al. Prognostic value of the association between MHC class I downregulation and PD-L1 upregulation in head and neck squamous cell carcinoma patients. Sci. Rep. 9, 7680 (2019).
Erdogdu, I. H. MHC class 1 and PDL-1 status of primary tumor and lymph node metastatic tumor tissue in gastric cancers. Gastroenterol. Res. Pract. (2019).
Bijen, C. B. M. et al. The prognostic role of classical and nonclassical MHC class I expression in endometrial cancer. Int. J. Cancer 126, 1417–1427 (2010).
Dibbern, M. E. et al. Loss of MHC class i expression in HPV-associated cervical and vulvar neoplasia: a potential mechanism of resistance to checkpoint inhibition. Am. J. Surg. Pathol. 44, 1184–1191 (2020).
Friedman L. A., Bullock T. N., Sloan E. A., Ring K. L., Mills A. M. MHC class I loss in endometrial carcinoma: a potential resistance mechanism to immune checkpoint inhibition. Mod. Pathol. 34, 627–636 (2021)
Abel, A. M., Yang, C., Thakar, M. S., Malarkannan, S. Natural killer cells: development, maturation, and clinical utilization. Front. Immunol. 9 (2018).
Hu, W., Wang, G., Huang, D., Sui, M., Xu, Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front. Immunol. 10 (2019).
Crowther, M. D. et al. Genome-wide CRISPR–Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat Immunol 21, 178–185 (2020).
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: Review conception: A.M; Draft paper preparation: A.M; Revisions and critical appraise for intellectual content: T.N.B., K.L.R. All authors reviewed and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mills, A.M., Bullock, T.N. & Ring, K.L. Targeting immune checkpoints in gynecologic cancer: updates & perspectives for pathologists. Mod Pathol 35, 142–151 (2022). https://doi.org/10.1038/s41379-021-00882-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00882-y
This article is cited by
-
Dynamic host immunity and PD-L1/PD-1 blockade efficacy: developments after “IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer”
British Journal of Cancer (2023)
-
IGSF11 and VISTA: a pair of promising immune checkpoints in tumor immunotherapy
Biomarker Research (2022)