Abstract
In 2018, the consensus meeting for the WHO Classification of Tumours of the Eye decided that conjunctival mucoepidermoid carcinoma should be reclassified as adenosquamous carcinoma, as this represented a better morphological fit. To examine the applicability of this terminology, we studied the clinical, histopathological, immunohistochemical and molecular pathology of 14 cases that were originally diagnosed as conjunctival mucoepidermoid carcinoma. There were 7 (50%) females and 7 (50%) males. The median age was 64 years. The left eye was affected in 8 and the right eye in 6 patients. In-situ carcinoma was present in 11/14 (79%) cases and comprised in-situ squamous cell carcinoma (SCC) and conjunctival intraepithelial neoplasia with mucinous differentiation (CIN-Muc). Invasive carcinoma was present in 11/14 (79%) cases. Group 1 (1/11 cases, 9%) comprised invasive SCC only. Group 2 (6/11 cases, 55%) comprised SCC with mucinous differentiation, manifesting as scattered intracellular mucin, occasionally together with intercellular mucin, with no evidence of true glandular differentiation. Group 3 (3/11 cases. 27%) comprised true adenosquamous carcinoma. Group 4 (1/11 cases, 9%) comprised pure adenocarcinoma. Thirteen of 14 cases (93%) underwent FISH for MAML2 translocation and none were rearranged. Two cases harboured high-risk HPV (type 16 and 18). The combined findings confirm that all lesions in our study were not mucoepidermoid carcinoma, but represented predominantly SCC with mucinous differentiation and adenosquamous carcinoma. We, therefore, recommend future revision of the WHO classification to include SCC with mucinous differentiation alongside adenosquamous carcinoma.
Similar content being viewed by others
Introduction
The term mucoepidermoid carcinoma (MEC), as applied to the conjunctiva, was first used in a publication by Rao and Font in 1976 [1]. The description was based on the diagnostic criteria from the original description of MEC of the salivary glands by Stewart et al. [2] Since then, publications listed in PUBMED have used this term to describe a conjunctival invasive tumour that is composed of two cell types: epidermoid cells and mucinous cells [1, 2]. However, MEC as applied to salivary glands is a distinct entity, comprising 3 cell types comprising epidermoid, intermediate and mucinous cells [3]. The 2017, 4th edition of the World Health Organisation (WHO) classification of head and neck tumours has a very useful table that compares the histology of adenosquamous and MEC. The table mentions: MEC has no evidence of origin from overlying squamous epithelium; shows no keratinization or keratin pearls; shows widespread glands with a lobular arrangement; the epidermoid and glandular cells are closely intermingled with lobules of tumour; it arises from submucosal glands; it contains intermediate cells and usually is associated with the MAML2 translocation [4]. In January 2018, the consensus and editorial meeting for the WHO Classification of Tumours of the Eye, met at the International Agency for Research into Cancer (IARC) in Lyon, France. During this meeting (at which one of the authors HSM was present), it was established that none of the papers published on conjunctival MEC could fulfil the strict histological criteria for true MEC as listed above. In the light of this, the consensus meeting agreed that conjunctival MEC be reclassified as adenosquamous carcinoma (ASC) [5].
This study will re-examine all published papers on conjunctival MEC and will discuss the applicability of the new WHO terminology, by examining the clinical, histopathological, immunohistochemical and molecular pathology of 14 cases that originally were diagnosed with conjunctival ‘MEC’.
Materials and methods
The study was approved by the Institute Research Boards (IRB) of the Royal Hallamshire Hospital Research Office, Sheffield UK and the Wills Eye Hospital Philadelphia USA. The Royal Hallamshire Hospital Sheffield pathology and clinical records (between 2001 and 2019) and the Wills Eye Hospital ophthalmic pathology and ocular oncology records (between 2003 and 2019) on all patients with conjunctival MEC were reviewed.
Clinical notes were retrieved and the following patient parameters were recorded for the study: age, sex, laterality, location of tumour (L/B = limbal/bulbar, F/T = fornix/tarsus, P/C = plica/caruncle, O = orbit), quadrant (U = superior, I = inferior, N = nasal, T = temporal), clinical size of the tumour (mm), duration of symptoms, presence/absence/nature of any prior treatment, type of definitive treatment, recurrence, time to first recurrence, presence and timing of metastatic disease, and follow up duration.
All tumours originally classified histopathologically as conjunctival MEC (in-situ or invasive) were re-assessed in the light of the WHO revised classification. The original haematoxylin and eosin (H&E) and mucin stained slides were reviewed by two ophthalmic pathologists (HSM and TM) and the following parameters were recorded: presence or absence of in-situ and invasive tumour, type of in-situ and invasive tumour, grade of invasive tumour, and presence or absence of perineural and lymphovascular space invasion. The grading of the invasive tumour was based on the AJCC histologic grading of conjunctival carcinoma [6].
Immunohistochemistry protocols
Each tumour was cut into 4 micron thick sections, collected on coated slides and exposed to the following immunohistochemical panel: CK7, CK17, BerEp4, p16, and Ki-67. The immunohistochemistry protocols are briefly as follows:
Royal Hallamshire Sheffield cases
CK7-Dako Copenhagen; ready to use antibody; Dako high pH retrieval buffer. CK17—Leica BioSystems, clone E3; ready to use antibody from the manufacturer; Antigen retrieval with Leica BioSystems ER2 buffer. BerEP4—Dako Copenhagen; ready to use antibody; Dako low pH retrieval buffer. p16—Roche UK; ready to use antibody; Roche CCI retrieval buffer. Ki-67—Dako Copenhagen; ready to use antibody; Dako low pH retrieval buffer.
Wills Eye Hospital Philadelphia cases
CK7-DAKO Carpinteria; ready to use antibody; Dako high pH retrieval buffer. CK17—Leica BioSystems, clone E3; ready to use antibody from the manufacturer; Antigen retrieval with Leica BioSystems ER2 buffer. BerEP4—Dako Carpinteria; 1:50 dilution; Dako low pH retrieval buffer, p16—Ventana Tucson; ready to use antibody; Ventana CCI retrieval buffer. Ki-67—Dako Carpinteria; ready to use antibody; Dako low pH retrieval buffer.
Immunohistochemistry assessment protocol
The antibody staining intensity was quantified as 0, 1+(mild), 2+(moderate), 3+(strong) and the percentage cells positive as 0, 1+(1–25%), 2+(26–50%), 3+(51–75%) and 4+(76–100%). For Ki-67 the absolute percentage positive cells were recorded.
Fluorescence in situ hybridization (FISH) analysis protocol
Royal Hallamshire Hospital and Wills Eye Hospital Cases: The MAML2 FISH assay was performed with the probe Zytolight SPEC MAML2 Dual Color Break Apart Probe (11q21) (ZytoVision, Bremerhaven, Germany) and the Histology FISH Accessory Kit (ZytoVision) in accordance with the manufacturer’s protocols. At least 40 randomly selected non-overlapping tumour cell nuclei were evaluated for the presence of yellow (normal) or green and red (chromosomal break-apart) fluorescent signals at 1000× magnification. The sample was considered positive for rearrangement when >20% of nuclei showed break-apart signals. CRTC1/3-MAML2 fusion gene-positive salivary gland MEC cases were used as a positive control. The MAML2 FISH assay was internally validated on 70 salivary gland MEC and 40 other (non-mucoepidermoid carcinoma) epithelial salivary gland tumours.
Human papilloma virus (HPV) analysis
For cases 4, 5, 12, 13, and 14, RNAscope (Leica BioSystems) for in situ detection of transcriptionally active high-risk HPV types 16, 18, 31, and 33 was performed on Bond III autostainer (Leica BioSystems) using “double Z” oligonucleotide probes conjugated to horseradish peroxidase (HRP) molecule for chromogenic reaction with 3,3′-diaminobenzidine (DAB). Appropriate positive and negative controls were run with all samples. Punctate brown staining in the tumour tissue was interpreted as positive.
For cases 6 and 13, HPV DNA in situ hybridization (ISH) was performed using ENZO probes (HPV genotypes 6, 11, 16, 18, 31, 33, 51; Enzo Life Sciences Framingham) according to the manufacturer’s instructions. Nuclear staining in the tumour tissue was interpreted as positive. Case 13 was also evaluated with RNAscope (see above).
For cases 1, 9, 10, and 11, analysis was performed at the Scottish HPV Reference Laboratory Edinburgh. Nucleic acid extraction was with the reagents within the DNA mini kit (Qiagen, Hilden, Germany) with a protocol adapted to maximise HPV nucleic acid recovery. DNA extracts were tested for HPV using the Optiplex HPV Genotyping Kit (Diamex GmbH, Heidelberg, Germany). This genotyping test detects 24 HPV types including all established high-risk types 6, 11,16, 18, 26, 31, 33, 35, 39, 42, 43, 44, 45, 51, 52, 53,56, 58, 59, 66, 68, 70, 73 and 82).
Results
Clinical data
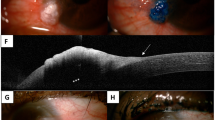
The clinical characteristics of the lesions are summarized in Table 1 and illustrated in Fig. 1. There were 7 females (50%) and 7 males (50%). The median age was 64 years (mean 66, range 54–86). The left eye was affected in 8 (57%) and the right eye in 6 (43%) cases. The tarsal and forniceal conjunctiva (9/14, 64%) in the inferior quadrant (7/14, 50%) was most commonly involved. Most tumours had nodular or multinodular architecture (9/14, 64%) and fleshy, pink-to-red colouration (11/12, 92%). Papillomatous appearance was noted in one tumour (1/14, 7%). Two lesions (2/12, 17%) were associated with surface leukoplakia. The mean maximum tumour dimension was 14 mm (median 13, range 3–30) and mean symptom duration was 9 months (median 6, range 3–24). Most common management was excision (with or without frozen section control of margins) and adjuvant cryotherapy (9/14, 64%). Two tumours failed topical and intralesional interferon (2/14, 14%) and were subsequently managed by excision with cryotherapy or by orbital exenteration (1/14, 7%). One patient with a recurrent pT4 tumour with perineural invasion was managed with adjuvant external beam radiotherapy (1/14, 7%). Recurrence was documented in 3/14 (21%) tumours 3, 9, and 12 months after treatment. Recurrence was associated with nodal metastasis in 2 of these 3 cases 3 and 30 months after treatment. All recurrent and metastasizing tumours were pT3a (one case) and pT4a (two cases). In contrast, 3 of 11 (27%) non-recurrent and non-metastasizing tumours were pT4.
a Case 9 showing diffuse red conjunctiva, corresponding histologically to in-situ carcinoma. The arrow indicates a subtle red thickening of the inferior tarsal conjunctiva, indicating the site of the invasive tumour. b Case 11. The medial scarring is from previous surgical excision with graft. The new invasive tumour is indicated by the arrow, showing lid margin white thickening and a raised red tarsal conjunctival area. c Case 10 showing a fleshy red, nodular gelatinous lesion over the medial bulbar conjunctiva with plica semilunaris involvement. d Case 8 showing a papillomatous, gelatinous mass with eyelid margin involvement in the inferotemporal forniceal and tarsal conjunctiva. e Case 7 showing a large, multinodular mass with erythematous base and surface leukoplakia involving the inferior tarsal and forniceal conjunctiva.
Histopathology and histochemical findings
Three of 14 (21%) cases showed in-situ carcinoma only, 3/14 (21%) cases showed invasive carcinoma only and 8/14 (57%) cases showed in situ and invasive carcinoma.
In-situ carcinoma component
In situ carcinoma was identified in 11/14 (79%) cases. It occurred as the sole lesion in 3/14 (21%) cases and was associated with invasive carcinoma in 8/14 (57%) cases.
Two of 11 (18%) cases comprised in situ squamous cell carcinoma (Fig. 2a), 7/11 (64%) cases were combination lesions of in situ SCC and dysplasia with mucinous differentiation and 2/11 (18%) cases were dysplasia with mucinous differentiation alone.
a Case 7 haematoxylin and eosin (H&E) showing typical keratinising in-situ squamous cell carcinoma. The top-right inset figure shows a higher power of the cytology and keratinisation. b Case 9 H&E showing conjunctival intraepithelial neoplasia with mucinous differentiation (CIN-Muc). The mucin is seen as intracytoplasmic light blue deposits throughout the full thickness of the dysplastic epithelium. This would be a ‘mucin-rich’ example. c Combined Alcian Blue/ Periodic Acid Schiff (AB/PAS) stain of CIN-Muc from Case 9, showing the numerous blue mucin deposits throughout the epithelium. The arrows indicate the basement membrane of the dysplastic epithelium. d Case 4. H&E showing CIN-Muc. The bottom left inset figure is a higher magnification showing occasional intracytoplasmic blue mucin deposits (white arrows). This is a ‘mucin-poor’ example of CIN-Muc. e AB stain of same case in Plate d showing the occasional blue intracytoplasmic mucin deposits within the CIN-Muc. The bottom left inset figure shows the nuclear atypia amongst which is an atypical nucleus on the right with intracytoplasmic mucin, showing that this cell is not an entrapped normal goblet cell. f Case 9 immunohistochemistry with CK7 showing uniform strong staining of the mucin-rich CIN-Muc. The inset figure shows CK17 immunostaining of CIN-Muc with just surface cell positivity. g Case 9 immunohistochemistry with CK17 showing in-situ SCC with extensive staining showing. In contrast, the CIN-Muc shows little staining (inset figure). h Case 9 immunohistochemistry with p16 showing block positivity of the mucin-rich CIN-Muc.
Dysplasia with mucinous differentiation is a descriptive term to denote mucin deposits within the cytoplasm of dysplastic cells or between the dysplastic cells. The extent of the mucin varied between cases. In mucin-rich cases, intracellular mucin was seen throughout the full-thickness of the dysplastic epithelium (Fig. 2b). The combined AB/PAS stains confirmed the extent of the intracytoplasmic mucin throughout the dysplastic epithelium (Fig. 2c). Mucin-poor cases comprised very occasional intracellular mucin deposits similar to goblet cells within the dysplastic epithelium (Figs. 2d, e and inset figure) with or without extracellular mucin deposits (not shown). Suitable terminologies for epithelial dysplasia with mucinous differentiation are debated in the discussion section of the paper.
CK7 stained the in-situ-disease moderately to strongly in cases where there was a mucin component (Fig. 2f) and the same area showed little CK17 staining (inset Fig. 2f). CK17 tended to be expressed by the cells that were squamous (Fig. 2g) with lower expression in the mucin rich areas (Fig. 2g inset). BerEP4 only stained 2/8 (25%) of the in-situ disease and both had a mucinous differentiation. P16 showed strong block positivity in 5/8 (63%) cases (Fig. 2h; block positivity is defined as continuous strong nuclear or nuclear plus cytoplasmic staining of the basal cell layer with extension upward involving at least one third of the epithelial thickness) and the Ki-67 rate was 50% or greater in 5/8 cases (63%) (not shown).
Invasive carcinoma component (11/14, 79%)
The invasive carcinomas were reclassified into four morphological groups.
Group 1 (1/11 cases, 9%) comprised invasive SCC only.
Group 2 (6/11 cases, 55%) comprised SCC with mucinous differentiation, manifesting as scattered intracellular mucin with or without intercellular mucin, with no evidence of true glandular differentiation.
Group 3 (3/11 cases, 27%) comprised true ASC composed of invasive SCC and adenocarcinoma components.
Group 4 (1/11 cases, 9%) comprised pure adenocarcinoma.
Four cases showed lymphovascular space invasion (the case from Group 1, 3 cases from Group 3) and two showed perineural invasion (one case from group 2 and one case from group 3).
The Group 1 case was invasive basaloid SCC (Fig. 3a), with no mucinous differentiation in the invasive component. However, the in-situ component showed mucinous differentiation.
a Case 4. H&E showing invasive basaloid variant SCC. Note the ribbons of basaloid cells with focal tumour necrosis (arrow). b Case 7. H&E showing typical invasive keratinising SCC. c Case 7. H&E. The arrow points to occasional goblet-cell like intracytoplasmic mucin deposits. d Case 7 Alcian blue (AB) stain, showing blue intracytoplasmic mucin deposits, confirming the diagnosis of invasive SCC with mucinous differentiation. e Case 9 H&E. The arrow points to invasive carcinoma arising from in-situ carcinoma indicated by the asterisk. f Case 9 H&E higher magnification showing signet ring-like spaces (arrows) in amongst non-keratinising SCC. g Case 9 AB stain showing that the signet ring-like spaces contain mucin, indicating a diagnosis of SCC with mucinous differentiation. h Case 11 H&E. The arrow points to the invasive adenocarcinoma part and the asterisk indicates the invasive SCC in this example of ASC. i Case 11 H&E showing the adenocarcinoma component that is well-differentiated, with back-to back neoplastic glands that contain eosinophilic secretion. j Case 11 H&E showing the non-keratinizing invasive SCC component. Note the ample pink cytoplasm. k Case 11 combined AB/Periodic acid Schiff stain showing pink mucin produced by the adenocarcinoma. l Case 12 H&E. This shows the keratinising SCC component (arrow points to keratin). m Case 12 H&E showing the confluent area of glandular differentiation component (arrow). n Case 14 H&E showing neoplastic glands only without a squamous carcinoma component. o Case 14 AB stain. This shows prominent mucin produced by the adenocarcinoma.
In Group 2 the squamous component was non-keratinising in 3 cases, non-keratinising with acantholysis in 2 cases and keratinising with acantholysis in 1 case (Fig. 3b). The mucin component manifested as scattered intracytoplasmic mucin deposits resembling goblet cells (Fig. 3c, d). In some cases; the mucin was seen in signet ring-like cells (Fig. 3e–g). No intermediate cells were identified in this group.
In Group 3 the cases comprised bone fide malignant glandular and malignant squamous components (Fig. 3h–m). The squamous component was non-keratinising in 2 cases (Fig. 3j) and keratinising in one case (Fig. 3l). The type and extent of the glandular component varied from case-to-case. In some cases, there were well-formed glands containing mucin (Fig. 3h–k) making up at least 50% of the tumour. In other cases, there was focal glandular differentiation comprising confluent groups of goblet cells (Fig. 3m). The glandular component tended to be side-by-side with the malignant squamous component. The malignant gland lumens contained mucin (Fig. 3k) and sometimes dirty necrotic debris. The cells lining the gland lumens were cytologically atypical. No case showed extravasation of mucin into the extracellular spaces, which is a characteristic feature of MEC of the salivary glands. No intermediate cells were identified in this group.
The Group 4 case showed a well-differentiated adenocarcinoma involving the caruncular stroma, with prominent goblet-like cells and a peripheral palisade of nuclei and prominent glandular differentiation containing ample luminal mucin (Fig. 3n, o). Although the neoplastic glandular structures contained epithelial cells with intercellular bridges, the solid SCC-type component was not identified. No keratinization was present. Intermediate cells were not conspicuous. The lesion was not associated with the surface epithelium, which lacked dysplasia
Table 2 summarises the above data.
Immunohistochemical findings
In Group 1 (1/11 cases), the invasive basaloid SCC was positive for CK7, BerEP4, and p16 (block positivity). It was negative for CK17. The Ki-67 proliferation index was 60%.
In Group 2 (6/11 cases), CK7 tended to stain the mucinous areas (Fig. 4a) with CK17 expressed at the periphery of the tumour lobules and in the more squamous rich areas (Fig. 4b). The Ki-67 proliferation index varied from 40 to 70% (average 50%) (Fig. 4c showing an ~50% proliferation fraction). P16 expression varied from no expression to block positivity (Fig. 4d). BerEP4 stained some cases diffusely with no preference for squamous or mucinous areas (not shown), with same cases showing no expression at all.
a Case 6. The morphological diagnosis was SCC with mucinous differentiation. CK7 stains an area with goblet cells. b Case 6. The same area as plate a showing peripheral CK17 staining of areas with squamous differentiation. c Case 6. Ki-67 showing around 50% proliferation fraction. d Case 6 showing p16 block positivity. e Case 11 where the morphological diagnosis was ASC. CK7 stains the adenocarcinoma component whereas the SCC component indicated by the asterisk is negative. f Case 11. CK17 stains the SCC component and was negative on the adenocarcinoma component (not shown as adenocarcinoma component was cutting out of section). g Case11. BerEP4 staining of the SCC component. h Case 11. Ki-67 staining of the SCC component. i Case 11. p16 stains the adenocarcinoma component and not the SCC component. j Case 14 where the morphological diagnosis was adenocarcinoma. This is the CK7 staining pattern showing strong staining in the neoplastic glands. k Case 14. CK17 showing staining of the edges of the neoplastic glands. l Case 14. BerEP4 showing staining of the neoplastic mucin-containing cells.
In Group 3 (3/11 cases) CK7 stained the adenocarcinoma areas (Fig. 4f) but also stained the entire tumour in some cases (not shown). CK17 tended to stain the squamous areas (Fig. 4f). The pattern of BerEP4 was variable from case to case, ranging from no expression through to staining one component (Fig. 4h) or both. The Ki-67 proliferation fraction (Fig. 4i) range was 20–60% (average 40%). The p16 staining pattern was moderate to strong/block positivity and in some cases also stained the adenocarcinoma component (Fig. 4j).
In Group 4 (1/11 cases) CK7 strained the goblet cell-rich areas (Fig. 4k) and CK17 stained the peripheral aspects of the adenocarcinoma islands (Fig. 4l). BerEP4 tended stained the goblet cell-rich areas (Fig. 4m). P16 showed areas with moderate to strong nuclear and cytoplasmic staining in >50–60% cells (not shown). The Ki-67 proliferation index was 10%.
In summary, there was no distinctive immunohistochemical pattern of CK7, CK17, BerEP4, p16, and Ki-67 expression that corresponded to the morphologic groupings or to the pTNM stage groupings in our cases.
Table 2 summarises the above data.
Molecular pathology findings
13 out of 14 cases underwent FISH to test for the presence or absence of MAML2 translocation. 13 out of 13 cases showed no MAML2 rearrangement (with the caveat of one case where the test was not performed).
There were 10 of 14 cases that were tested for HPV and only 2 showed evidence of HPV. Case 6 harboured HPV16 DNA (by in-situ hybridization) and case 9 harboured HPV18 DNA (in-situ and invasive components were submitted separately and both were positive HPV 18 DNA by PCR testing).
Table 2 summarizes the above data.
Discussion
Stewart et al. coined the term ‘mucoepidermoid tumour’ in a report published in 1945 in the American Journal of Surgery [2]. He described tumours with two components: squamoid and mucinous. The current World Health Organisation (WHO) definition of MEC, when applied to salivary glands is ‘a distinctive salivary gland malignancy composed of mucinous, intermediate (clear cell) and squamoid tumour cells forming cystic and solid patterns [3].
‘MEC’ of the conjunctiva was described by Rao and Font in 1976 as a series of 5 cases [1]. Their 5 cases were composed of ‘epidermoid cells’ and showed focal mucin production either in cystic spaces or within signet ring-like cells. Two cases showed a distinct biphasic adenocarcinoma and squamous carcinoma mix where the respective components were side-by-side. So, while at the time, Rao and Font were correct in designating their 5 cases as ‘mucoepidermoid’ based on the diagnostic criteria of Stewart et al. [2], more recent authoritative references imply that their 5 cases are not true MEC. We found that our 14 cases that had been designated as MEC, could be reclassified into 4 categories, with the bulk of the tumours falling into SCC with mucinous differentiation (Group 2) and ASC (Group 3). The 5 cases of Rao and Font actually fall into the same categories: SCC with mucinous differentiation (3/5 cases), corresponding to group 2 and ASC (2/5 cases), corresponding to group 3. MEC was excluded on the basis of origin from the conjunctival epithelium (MEC does not take origin from overlying squamous epithelium yet Rao and Font state in their paper that the 5 cases arose from the epithelium), and the absence of intermediate type cells in the invasive tumour.
SCC with mucinous differentiation is not a novel idea or terminology that we are using in this study. Basaloid SCC of the head and neck can feature focal intracytoplasmic mucin deposits [7], oesophageal squamous carcinoma is known to show focal mucinous differentiation [8, 9] and in the skin, the term SCC with mucinous metaplasia has been reported [10, 11]. Scrutiny of all relevant publications of conjunctival MEC subsequent to Rao and Font’s paper [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29] indicates the histological diagnoses all fall into the categories of SCC with mucinous differentiation and ASC. For example, the publication by Jastrzebski clearly mentions the presence of tumour keratinisation, which is never a feature of MEC, yet is readily found in SCC and ASC [24]. Some authors have expressed their scepticism about ‘conjunctival MEC’ terminology over the years by stating that it is a variant of SCC [30,31,32] and there is one publication by Kase et al. that clearly uses the term conjunctival ASC over MEC [33]. The figures from the latter case clearly show invasive SCC and adenocarcinoma components side-by-side. The WHO Classification of Head and Neck Tumours defines ASC as ‘a malignant tumour that arises from the surface epithelium and shows both squamous and glandular differentiation’. The histological description clearly mentions biphasic morphology with distinct squamous and adenocarcinoma components in close proximity. The glandular component is said to occur more commonly in the deeper parts of the tumour and can be cribriform or tubulo-glandular with mostly intraluminal and occasional intracytoplasmic mucin [3]. The authors of this particular chapter provide a very clearly laid out table that compares the differences between head and neck adenosquamous and MEC. ASC shows origin from overlying epithelium (dysplasia), shows keratinization and keratin pearls in the squamous component, has no intermediate cells and does not harbour the MAML2 translocation [3]. All of these features were noted in our cases of ASC of the conjunctiva and most of these features are present in some of the cases that were designated as conjunctival MEC, in the literature alluded to above. What is further clarified by the WHO approach is that squamous cell carcinoma with mucinous differentiation is not designated as ASC since definite neoplastic glands need to be present. Recently, other anatomical sites, including pancreas and nasolacrimal sac, have been subject to similar tumour re-classifications and demonstrated that MEC in these sites is not a counterpart of CRTC1/3-MAML2 fusion gene-related salivary gland MEC, leading to re-designation of these tumours as ASC [34, 35].
Only 2 previous studies have examined the role of immunohistochemistry in the diagnosis of conjunctival ‘MEC’. Rankin et al. equated the expression of CK7 to a diagnosis of ‘MEC’ rather than SCC in a single case report [36]. A more comprehensive study by Jastrzebski et al. examined 8 specimens from 4 patients in an attempt to distinguish MEC from SCC using histochemical mucin stains and a panel of antibodies comprising BRST-1, CEA, CK-7, EMA, HMWK, LMWK and mucin-1 [26]. They concluded that histochemical mucin stains were the most sensitive in distinguishing SCC from MEC and in cases where the mucin stains were inconclusive, immunohistochemical stains for CEA and mucin-1 were helpful to make the distinction. The cases described in Jastrzebski et al’s series can be reclassified as SCC with mucinous differentiation as defined in our series- and are not MECs. That study actually was making the distinction between conventional SCC and SCC with mucinous differentiation. Furthermore, CEA and Mucin-1 are markers that are known to be expressed by a variety of SCCs from various organ sites [37, 38]. In our study we utilized CK7, BerEP4 CK17, p16 and Ki-67. CK7 and BerEP4 were selected on the basis of their bias to stain glandular malignancy [39, 40], CK17 was selected for its bias to stain head and neck squamous malignancies [41]. A combination of p16 and Ki-67 was selected as surrogate markers to ascertain whether they would indicate a potential HPV etiology [42, 43]. We found that mucin stains clearly identified intracellular and extracellular mucin in groups 2, 3 and 4 tumours, identical to the observations of Jastrzebski et al. [26]. While there was a trend for CK7 to stain mucinous and glandular areas and CK17 to stain more squamous areas (except for Group 1), BerEP4 did not reveal a distinct enough pattern amongst the different groups. Also block-like p16 expression and brisk Ki-67 expression did not equate in the majority of cases to the presence of HPV DNA.
Thirteen of the cases in our series (with the caveat of one case where the test was not performed), did not harbour the gene rearrangement involving MAML2 that is found in 75 to 80% of salivary MEC’s [44,45,46,47,48,49], providing further evidence that the conjunctiva does not harbour a CRTC1/3-MAML2 fusion gene-related MEC counterpart. A recent study on head and neck SCC and ASC showed no evidence of gene rearrangement harbouring MAML2 either [50]. This molecular evidence entirely supports the morphological reclassification into SCC with mucinous differentiation and ASC and parallels well the evidence from other organ system tumour re-classifications [34, 35].
Two cases in our series contained HPV DNA (16 and 18). Both cases were SCC with mucinous differentiation and displayed strong block p16 staining throughout the in-situ and invasive carcinoma components. The case harbouring HPV18 had separate samples of the in-situ and invasive disease submitted for analysis and both contained HPV18 DNA. Interestingly, two of three cases that recurred were HPV positive and both metastasised, whereas all of the other tumours were HPV DNA negative. The role of HPV infection in the head and neck SCC is well-documented. Twelve percent of pharyngeal SCC, 3% of oral SCC, and 30–60% of oropharyngeal SCC cases are caused by HPV infection [51]. It is generally accepted that the presence of high-risk HPV in head and neck squamous carcinoma is associated with a favourable prognosis [52, 53]. Head and neck ASC also harbour high risk HPV types with positivity rates of 16% [54] with a more favourable prognosis compared to HPV negative cases. However, recently, it has been established that a subset of conjunctival in-situ SCC that harbour high-risk HPV type 16 have a higher local recurrence rate [55] suggesting that high-risk HPV presence in conjunctival lesions may predict a worse prognosis and therefore could be a group that are an exception to the rule. Notably, most of the block positive p16 positive cases did not harbour HPV DNA, suggesting that the rest of the cases may have alternative aetiologies. An important consideration is the technique that is used to detect HPV in paraffin tissues. The presence of HPV DNA doesn’t necessarily mean that it is transcriptionally active and preferably DNA detection techniques should be backed up by RNAscope, a recently developed highly sensitive assay to detect transcriptionally active high risk HPV RNA [56]. The RNAscope negative results in our study are highly supportive of a genuine lack of transcriptionally active HPV RNA, therefore arguing against HPV causation. However, the 2 cases that revealed high risk HPV DNA by PCR and in-situ hybridization showed block positivity with p16 immunohistochemistry, arguing that HPV was genuinely present and not a contaminant and was probably driving the neoplastic process. It would have been useful to employ RNAscope on those cases that were HPV DNA negative as some of these may have been false negatives due to technique limitations [56].
In regards to the in-situ component in this study, previously Margo and Groden described the case of a 66-year-old female with a papillary lesion at the 7 o’clock corneo-scleral limbus [57]. The histology showed dysplastic cells occupying at least two-thirds of the epithelium with cytoplasmic mucin deposits present at all levels of the epithelium. Normal goblet cells were absent from the lesion. They used the term ‘squamous epithelial dysplasia with mucoepidermoid features’. Joang et al. have made recent identical observations in an 86-year-old male with a unilateral lesion at the supero-temporal limbus and called it ‘CIN with mucoepidermoid differentiation’ [58]. Both papers stressed that the in-situ disease could be the precursor of invasive conjunctival ‘MEC’.
In the cervix, the term ‘stratified mucin producing intraepithelial lesion (SMILE) is used to describe a lesion which is composed of full thickness atypical epithelial cells with mucin vacuoles throughout, also referred to as in-situ ASC [59]. SMILE can be associated with typical squamous cervical intraepithelial neoplasia (CIN), adenocarcinoma in situ and with invasive SCC, ASC and adenocarcinoma [59]. Recently, it has been shown to be an HPV driven process, expressing block p16 expression and harbouring high risk HPV [60]. In the vulva, a similar lesion has been designated vulval intraepithelial neoplasia with mucinous differentiation [61]. Taking note of terminology from these various anatomical sites, we have chosen the term conjunctival intraepithelial neoplasia with mucinous differentiation (CIN-Muc) to describe the lesion in the conjunctiva that resembles SMILE of the cervix. CIN-Muc can be mucin-rich or mucin-poor as described for SMILE of the cervix [62]. Interestingly, one of the cases of CIN-Muc from our series expressed block p16 positivity and harboured HPV18 by PCR, similar to cervical SMILE. However, that case was in the minority, as the other cases of p16 expressing CIN-Muc did not harbour HPV, although this could have been due to sensitivity limitations of the different assays utilised for the detection of HPV in this study.
In summary, we have shown that the vast majority of cases that were called conjunctival MEC in the literature can be re-classified as SCC with mucinous differentiation (with no true malignant glands present) or ASC. In 13 of 13 cases evaluated in our series, there was no gene fusion involving MAML2 and only 2 cases showed high-risk HPV DNA. We have devised new terminology to describe conjunctival intraepithelial neoplasia that elaborates mucin, preferring the term conjunctival intraepithelial neoplasia with mucinous differentiation (CIN-Muc) further qualified as mucin-rich and mucin-poor in line with the terminology already established for cervical SMILE, with which CIN-Muc shares similarity.
Finally, the current WHO Classification of Eye Tumours designation of all conjunctival MEC as ASC represents, in our view, a positive step in the right direction, but is an oversimplification. We recommend that the next edition (5th edition) permit the accommodation of SCC with mucinous differentiation to reflect more accurately the morphological observations made in this study, the review of the literature and feedback from colleagues.
References
Rao NA, Font RL. Mucoepidermoid carcinoma of the conjunctiva: a clinicopathologic study of five cases. Cancer. 1976;38:1699–709.
Stewart FW, Foote FW, Becker WF. Mucoepidermoid tumors of salivary glands. Am J Surg. 1945;122:820–44.
Brandwein-Gensler M, Bell D, Inagaki H, Katabi N, Leivo I, Seethala R, et al. Mucoepidermoid Carcinoma. In: El-Naggar ChanJKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. (4th Edition). Lyon: IARC; 2017. p. 163–4.
Prasad MI, Cardesa A, Helliwell T, Hille J, Nadal A. Adenosquamous Carcinoma. In: El-Naggar ChanJKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. 4th Edition. Lyon: IARC; 2017. p. 89.
Mudhar HS, Edward DP, Chan ASY. Adenosquamous carcinoma of the conjunctiva and caruncle. In: Grossniklaus HE, Eberhart CG, Kivela TT, eds. WHO classification of tumours of the eye. 4th Edition. Lyon: IARC; 2018. p. 20.
Conway MR, Graue GF, Pelayes D, Pe’er J, Wilson MW, Wittekind CW, et al. Conjunctival Carcinoma. In: Amin MB, ed. AJCC Cancer Staging Manual. 8th Edition. Basel: Springer; 2017. p. 787–93.
Cho KJ, Jeong SU, Kim SB, Lee SW, Choi SH, Nam SY, et al. Basaloid squamous cell carcinoma of the head and neck: subclassification into basal, ductal, and mixed subtypes based on comparison of clinico-pathologic features and expression of p53, cyclin D1, epidermal growth factor receptor, p16, and human papillomavirus. J Pathol Transl Med. 2017;51:374–80.
Lam KY, Dickens P, Loke SL, Fok M, Ma L, Wong J. Squamous cell carcinoma of the oesophagus with mucin-secreting component (muco-epidermoid carcinoma and adenosquamous carcinoma): a clinicopathologic study and a review of literature. Eur J Surg Oncol. 1994;20:25–31.
Lam KY, Loke SL, Ma LT. Histochemistry of mucin secreting components in mucoepidermoid and adenosquamous carcinoma of the oesophagus. J Clin Pathol. 1993;46:1011–5.
Caputo V, Colombi R, Ribotta M, Rongioletti F. Cutaneous squamous cell carcinoma with mucinous metaplasia on the sole associated with high-risk human papillomavirus type 18. Am J Dermatopathol. 2011;33:317–22.
Friedman KJ, Hood AF, Farmer ER. Cutaneous squamous cell carcinoma with mucinous metaplasia. J Cutan Pathol. 1988;15:176–82.
Brownstein S. Mucoepidermoid carcinoma of the conjunctiva with intraocular invasion. Ophthalmology 1981;88:1226–30.
Searl SS, Krigstein HJ, Albert DM, Grove AS Jr. Invasive squamous cell carcinoma with intraocular mucoepidermoid features. conjunctival carcinoma with intraocular invasion and diphasic morphology. Arch Ophthalmol. 1982;100:109–11.
Herschorn BJ, Jakobiec FA, Hornblass A, Iwamoto T, Harrison WG. Mucoepidermoid carcinoma of the palpebral mucocutaneous junction. a clinical, light microscopic and electron microscopic study of an unusual tubular variant. Ophthalmology 1983;90:1437–46.
Gamel JW, Eiferman RA, Guibor P. Mucoepidermoid carcinoma of the conjunctiva. Arch Ophthalmol 1984;102:730–1.
Margo CE, Weitzenkorn DE. Mucoepidermoid carcinoma of the conjunctiva: report of a case in a 36-year-old with paranasal sinus invasion. Ophthalmic Surg 1986;17:151–4.
Carrau RL, Stillman E, Canaan RE. Mucoepidermoid carcinoma of the conjunctiva. Ophthalmic Plast Reconstr Surg. 1994;10:163–168.
Biswas J, Datta M, Subramaniam N. Mucoepidermoid carcinoma of the conjunctiva of the lower lid-report of a case. Indian J Ophthalmol. 1996;44:231–3.
Yoon YD, Grossniklaus H. Tumors of the cornea and conjunctiva. Curr Opin Ophthalmol. 1997;8:55–58.
Gündüz K, Shields CL, Shields JA, Mercado G, Eagle RC Jr. Intraocular neoplastic cyst from mucoepidermoid carcinoma of the conjunctiva. Arch Ophthalmol 1998;116:1521–3.
Soong HK, Feil SH, Elner VM, Elner S, Flint A. Conjunctival mucoepidermoid carcinoma in a young HIV-infected man. Am J Ophthalmol. 1999;128:640–3.
Hwang IP, Jordan DR, Brownstein S, Gilberg SM, McEachren TM, Prokopetz R. Mucoepidermoid carcinoma of the conjunctiva: a series of three cases. Ophthalmology 2000;107:801–5.
Panda A, Sharma N, Sen S, Ray M. Mucoepidermoid carcinoma of the conjunctiva managed by frozen section-guided excision and lamellar keratoplasty. Clin Exp Ophthalmol. 2003;31:275–7.
Kapur R, Sugar J, Edward DP. Conjunctival mucoepidermoid carcinoma: clear cell variant. Arch Ophthalmol 2005;123:1265–8.
Robinson JW, Brownstein S, Jordan DR, Hodge WG. Conjunctival mucoepidermoid carcinoma in a patient with ocular cicatricial pemphigoid and a review of the literature. Surv Ophthalmol 2006;51:513–9.
Jastrzebski A, Brownstein S, Jordan DR, Gilberg SM. Histochemical analysis and immunohistochemical profile of mucoepidermoid carcinoma of the conjunctiva. Saudi J Ophthalmol. 2012;26:205–10.
Moloney TP, Trinh T, Farrah JJ. A case of conjunctival mucoepidermoid carcinoma in Australia. Clin Ophthalmol 2014;8:11–14.
Rishi P, Sharma R, Subramanian K, Subramanian M. Mucoepidermoid carcinoma of the conjunctiva with lung metastasis. Indian J Ophthalmol. 2015;63:457–9.
Dutta B, Biswas N, Roy R, Debb AR. Primary mucoepidermoid carcinoma of the bulbar conjunctiva. J Cancer Res Ther. 2019;15:712–4.
Grossniklaus HE, Green WR, Luckenbach M, Chaan CC. Conjunctival lesions in adults. a clinical and histopathologic review. Cornea 1987;6:78–116.
Cervantes G, Rodríguez AA Jr, Leal AG. Squamous cell carcinoma of the conjunctiva: clinicopathological features in 287 cases. Can J Ophthalmol. 2002;37:14–19.
Mittal R, Rath S, Vemuganti GK. Ocular surface squamous neoplasia—review of etio-pathogenesis and an update on clinico-pathological diagnosis. Saudi J Ophthalmol. 2013;27:177–86.
Kase S, Yoshikawa H, Nakajima Y, Noda M, Ishida S. Adenosquamous carcinoma of the conjunctiva: a case report. Oncol Lett. 2014;7:1941–3.
Saeki K, Ohishi Y, Matsuda R, et al. “Pancreatic Mucoepidermoid Carcinoma” Is not a Pancreatic Counterpart of CRTC1/3-MAML2 Fusion Gene-related Mucoepidermoid Carcinoma of the Salivary Gland, and May More Appropriately Be Termed Pancreatic Adenosquamous Carcinoma With Mucoepidermoid Carcinoma-like Features. Am J Surg Pathol. 2018;42:1419–28.
Gervasio KA, Zhang PJL, Penne RB, Stefanyszyn MA, Eagle RC Jr, Puthiyaveettil R, et al. Mucoepidermoid Carcinoma of the Lacrimal Sac: Clinical-Pathologic Analysis, Including Molecular Genetics. Ocular Oncology and Pathology Published online: September 20, 2019. https://doi.org/10.1159/000502699.
Rankin JK, Jakobiec FA, Zakka FR, Fosyer CS. An improved approach to diagnosing and treating conjunctival mucoepidermoid carcinoma. Surv Ophthalmol. 2012;57:337–46.
Ordóñez NG. The diagnostic utility of immunohistochemistry in distinguishing between epithelioid mesotheliomas and squamous carcinomas of the lung: a comparative study. Mod Pathol. 2006;19:417–28.
Croce MV, Rabassa ME, Price MR, Segal-Eiras A. MUC1 mucin and carbohydrate associated antigens as tumor markers in head and neck squamous cell carcinoma. Pathol Oncol Res. 2001;7:284–91.
Maniar KP, Umphress B. Stains, Cytokeratin 7 (CK7, K7) [pathologyoutlines Web site]. January 28, 2019. Available at: http://www.pathologyoutlines.com/topic/stainsck7.html. Accessed August 29, 2019.
Pernick Nat. Stains, BerEP4 / EpCAM [pathologyoutlines Web site]. March 4, 2019. Available at: https://www.pathologyoutlines.com/topic/stainsepcam.html. Accessed August 29, 2019.
Sesterhenn AM, Mandic R, Dünne AA, Werner JA. Cytokeratins 6 and 16 are frequently expressed in head and neck squamous cell carcinoma cell lines and fresh biopsies. Anticancer Res 2005;25:2675–80.
El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2006;101:339–45.
El-Mofty SK. Human papillomavirus (HPV) related carcinomas of the upper aerodigestive tract. Head Neck Pathol. 2007;1:181–5.
Behboudi A, Enlund F, Winnes M, Andrén Y, Nordkvist A, Leivo I, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–81.
Chiosea SI, Dacic S, Nikiforova MN, Seethala RR. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope. 2012;122:1690–4.
Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 tr6nslocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34:1106–21.
Garcia JJ, Hunt JL, Weinreb I, McHugh JB, Barnes EL, Cieply K, et al. Fluorescence in situ hybridization for detection of MAML2 rearrangements in oncocytic mucoepidermoid carcinomas: utility as a diagnostic test. Hum Pathol. 2011;42:2001–9.
Okabe M, Miyabe S, Nagatsuka H, Terada A, Hanai N, Yokoi M, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12:3902–7.
Luk PP, Wykes J, Selinger CI, Ekmejian R, Tay J, Gao K, et al. Diagnostic and prognostic utility of Mastermind-like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radio. 2016;121:530–41.
Kass JI, Lee SC, Abberbock S, Seethala RR, Duvvuri U. Adenosquamous carcinoma of the head and neck: molecular analysis using CRTC-MAML FISH and survival comparison with paired conventional squamous cell carcinoma. Laryngoscope 2015;125:E371–376.
Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(Suppl. 3):S11–S25.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl J Med. 2010;363:24–35.
Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9.
Masand RP, El-Mofty SK, Ma XJ, Luo Y, Flanagan JJ, Lewis JS Jr. Adenosquamous carcinoma of the head and neck: relationship to human papillomavirus and review of literature. Head Neck Pathol 2011;5:108–16.
Griffin H, Mudhar HS, Rundle P, Shiraz A, Mahmood R, Egawa N, et al. Human Papillomavirus type 16 causes a defined subset of conjunctival in-situ squamous cell carcinomas. Mod. Pathol. 2019;33:74–90.
Bussu F, Ragin C, Boscolo-Rizzo P, Rizzo D, Gallus R, Delogu G, et al. HPV as a marker for molecular characterization in head and neck oncology: Looking for a standardization of clinical use and of detection method(s) in clinical practice. Head Neck. 2019;41:1104–11.
Margo CE, Groden LR. Intraepithelial neoplasia of the conjunctiva with mucoepidermoid differentiation. Am J Ophthalmol. 1989;108:600–1.
Joag MG, Gupta A, Galor A, Dubovy SR, Bermudez-Magner JA, Wang J, et al. Conjunctival intraepithelial neoplasia with mucoepidermoid differentiation: a case report of a subtle lesion. Ocul Oncol Pathol. 2015;1:278–82.
Park JJ, Sun D, Quade BJ, Flynn C, Sheets EE, Yang A, et al. Stratified mucin-producing intraepithelial lesions of the cervix: adenosquamous or columnar cell neoplasia? Am J Surg Pathol. 2000;24:1414–9.
Fukui S, Nagasaka K, Iimura N, Kanda R, Ichinose T, Sugihara T, et al. Detection of HPV RNA molecules in stratified mucin-producing intraepithelial lesion (SMILE) with concurrent cervical intraepithelial lesion: a case report. Virol J. 2019;16:76.
McCluggage WG, Jamison J, Boyde A, Ganesan R. Vulval intraepithelial neoplasia with mucinous differentiation: report of 2 cases of a hitherto undescribed phenomenon. Am J Surg Pathol. 2009;33:945–9.
Lastra RR, Park KJ, Schoolmeester JK. Invasive stratified mucin-producing carcinoma and stratified mucin-producing intraepithelial lesion (SMILE): 15 Cases presenting a spectrum of cervical neoplasia with description of a distinctive variant of invasive adenocarcinoma. Am J Surg Pathol. 2016;40:262–9.
Funding
This work was supported in part by the Filkins Family Foundation, Council Bluffs, IA, USA. The UK part of the study did not receive any specific funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mudhar, H.S., Milman, T., Zhang, P.J.L. et al. Conjunctival ‘mucoepidermoid carcinoma’ revisited: a revision of terminology, based on morphologic, immunohistochemical and molecular findings of 14 cases, and the 2018 WHO Classification of Tumours of the Eye. Mod Pathol 33, 1242–1255 (2020). https://doi.org/10.1038/s41379-020-0456-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0456-9