Abstract
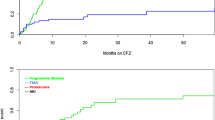
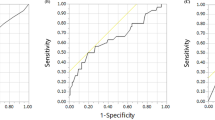
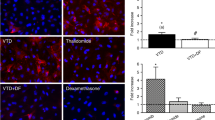
Carfilzomib (CFZ) improves survival in relapsed/refractory multiple myeloma but is associated with cardiovascular adverse events (CVAEs). We prospectively investigated the effect of CFZ on endothelial function and associations with CVAEs. Forty-eight patients treated with Kd (CFZ 20/56 mg/m2 and dexamethasone) underwent serial endothelial function evaluation, using brachial artery flow-mediated dilatation (FMD) and 26S proteasome activity (PrA) measurement in PBMCs; patients were followed until disease progression or cycle 6 for a median of 10 months. FMD and PrA decreased acutely after the first dose (p < 0.01) and FMD decreased at cycles 3 and 6 compared to baseline (p ≤ 0.05). FMD changes were associated with CFZ-induced PrA changes (p < 0.05) and lower PrA recovery during first cycle was associated with more prominent FMD decrease (p = 0.034 for group interaction). During treatment, 25 patients developed Grade ≥3 CVAEs. Low baseline FMD (HR 2.57 lowest vs. higher tertiles, 95% CI 1.081–6.1) was an independent predictor of CVAEs. During treatment, an acute FMD decrease >40% at the end of first cycle was also independently associated with CVAEs (HR = 3.91, 95% CI 1.29–11.83). Kd treatment impairs endothelial function which is associated with PrA inhibition and recovery. Both pre- and posttreatment FMD predicted CFZ-related CVAEs supporting its role as a possible cardiovascular toxicity biomarker.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ziogas DC, Terpos E, Kastritis E, Dimopoulos MA. An overview of the role of carfilzomib in the treatment of multiple myeloma. Expert Opin Pharmacother. 2017;18:1883–97.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2014;372:142–52.
Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng WJ, Oriol A, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–37.
Shah C, Bishnoi R, Jain A, Bejjanki H, Xiong S, Wang Y, et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma. 2018;59:2557–69.
Jain T, Narayanasamy H, Mikhael J, Reeder CB, Bergsagel PL, Mayo A, et al. Systolic dysfunction associated with carfilzomib use in patients with multiple myeloma. Blood Cancer J. 2017;7:642.
Bringhen S, Milan A, Ferri C, Wäsch R, Gay F, Larocca A, et al. Cardiovascular adverse events in modern myeloma therapy—incidence and risks. A review from the European Myeloma Network (EMN) and Italian Society of Arterial Hypertension (SIIA). Haematologica. 2018;103:1422–32.
Chen JH, Lenihan DJ, Phillips SE, Harrell SL, Cornell RF. Cardiac events during treatment with proteasome inhibitor therapy for multiple myeloma. Cardio-Oncology. 2017;3:4.
Dimopoulos MA, Roussou M, Gavriatopoulou M, Psimenou E, Ziogas D, Eleutherakis-Papaiakovou E, et al. Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv. 2017;1:449–54.
Yui JC, Van Keer J, Weiss BM, Waxman AJ, Palmer MB, D’Agati VD, et al. Proteasome inhibitor associated thrombotic microangiopathy. Am J Hematol. 2016;91:E348–52.
Poredos P, Jezovnik MK. Endothelial dysfunction and venous thrombosis. Angiology. 2017;69:564–7.
Hobeika L, Self SE, Velez JC. Renal thrombotic microangiopathy and podocytopathy associated with the use of carfilzomib in a patient with multiple myeloma. BMC Nephrol. 2014;15:156.
Rosenthal A, Luthi J, Belohlavek M, Kortüm KM, Mookadam F, Mayo A, et al. Carfilzomib and the cardiorenal system in myeloma: an endothelial effect? Blood Cancer J. 2016;6:e384.
Stangl K, Stangl V. The ubiquitin-proteasome pathway and endothelial (dys)function. Cardiovascular Res. 2009;85:281–90.
Meiners S, Ludwig A, Stangl V, Stangl K. Proteasome inhibitors: poisons and remedies. Med Res Rev. 2008;28:309–27.
Brandes Ralf P. Endothelial dysfunction and hypertension. Hypertension. 2014;64:924–8.
Lerman A, Zeiher, Andreas M. Endothelial function. Circulation. 2005;111:363–8.
Chen-Scarabelli C, Corsetti G, Pasini E, Dioguardi FS, Sahni G, Narula J, et al. Spasmogenic effects of the proteasome inhibitor carfilzomib on coronary resistance, vascular tone and reactivity. EBioMedicine. 2017;21:206–12.
Lorenz M, Wilck N, Meiners S, Ludwig A, Baumann G, Stangl K, et al. Proteasome inhibition prevents experimentally-induced endothelial dysfunction. Life Sci. 2009;84:929–34.
Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768.
Lendvai N, Tsakos I, Devlin SM, Schaffer WL, Hassoun H, Lesokhin AM, et al. Predictive biomarkers and practical considerations in the management of carfilzomib-associated cardiotoxicity. Leuk Lymphoma. 2018;59:1981–5.
Iannaccone A, Bruno G, Ravera A, Gay F, Salvini M, Bringhen S, et al. Evaluation of cardiovascular toxicity associated with treatments containing proteasome inhibitors in multiple myeloma therapy. High Blood Press Cardiovasc Prev. 2018;25:209–18.
Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. 2019;37:1946–55.
Stamatelopoulos K, Georgiopoulos G, Athanasouli F, Nikolaou PE, Lykka M, Roussou M, et al. Reactive vasodilation predicts mortality in primary systemic light-chain amyloidosis. Circ Res. 2019;125:744–58.
Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5.
Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–9.
Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90:40l–8l.
Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–74.
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65.
Mancini GB, Yeoh E, Abbott D, Chan S. Validation of an automated method for assessing brachial artery endothelial dysfunction. Can J Cardiol. 2002;18:259–62.
Preik M, Lauer T, Heiss C, Tabery S, Strauer BE, Kelm M. Automated ultrasonic measurement of human arteries for the determination of endothelial function. Ultraschall Med. 2000;21:195–8.
Tsakiri EN, Terpos E, Papanagnou ED, Kastritis E, Brieudes V, Halabalaki M, et al. Milder degenerative effects of Carfilzomib vs. Bortezomib in the Drosophila model: a link to clinical adverse events. Sci Rep. 2017;7:17802.
Papanagnou ED, Terpos E, Kastritis E, Papassideri IS, Tsitsilonis OE, Dimopoulos MA, et al. Molecular responses to therapeutic proteasome inhibitors in multiple myeloma patients are donor-, cell type- and drug-dependent. Oncotarget. 2018;9:17797–809.
Tsakiri EN, Sykiotis GP, Papassideri IS, Gorgoulis VG, Bohmann D, Trougakos IP. Differential regulation of proteasome functionality in reproductive vs. somatic tissues of Drosophila during aging or oxidative stress. Faseb J. 2013;27:2407–20.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81.
Fotiou D, Roussou M, Gakiopoulou C, Psimenou E, Gavriatopoulou M, Migkou M, et al. Carfilzomib associated renal toxicity is common and unpredictable: a comprehensive analysis of 114 multiple myeloma patients. Blood Cancer J. 2020;10:109. https://doi.org/10.1038/s41408-020-00381-4.
Berenson JR, Cartmell A, Bessudo A, Lyons RM, Harb W, Tzachanis D. et al. CHAMPION-1: a phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood. 2016;127:3360–8. https://doi.org/10.1182/blood-2015-11-683854.
Efentakis P, Kremastiotis G, Varela A, Nikolaou PE, Papanagnou ED, Davos CH, et al. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood. 2019;133:710–23.
Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–69.
Bonetti Piero O, Lerman Lilach O, Lerman A. Endothelial dysfunction. Arteriosclerosis, Thrombosis, Vasc Biol. 2003;23:168–75.
Migliacci R, Becattini C, Pesavento R, Davi G, Vedovati MC, Guglielmini G, et al. Endothelial dysfunction in patients with spontaneous venous thromboembolism. Haematologica. 2007;92:812–8.
Gavazzoni M, Lombardi CM, Vizzardi E, Gorga E, Sciatti E, Rossi L, et al. Irreversible proteasome inhibition with carfilzomib as first line therapy in patients with newly diagnosed multiple myeloma: early in vivo cardiovascular effects. Eur J Pharm. 2018;838:85–90.
Protogerou AD, Sfikakis PP, Stamatelopoulos KS, Papamichael C, Aznaouridis K, Karatzis E, et al. Interrelated modulation of endothelial function in Behcet’s disease by clinical activity and corticosteroid treatment. Arthritis Res Ther. 2007;9:R90.
Gonzalez-Juanatey C, Llorca J, Garcia-Porrua C, Sanchez-Andrade A, Martín J, Gonzalez-Gay MA. Steroid therapy improves endothelial function in patients with biopsy-proven giant cell arteritis. J Rheumatol. 2006;33:74–8.
Iuchi T, Akaike M, Mitsui T, Ohshima Y, Shintani Y, Azuma H, et al. Glucocorticoid excess induces superoxide production in vascular endothelial cells and elicits vascular endothelial dysfunction. Circ Res. 2003;92:81–7.
Mangos GJ, Walker BR, Kelly JJ, Lawson JA, Webb DJ, Whitworth JA. Cortisol inhibits cholinergic vasodilatation in the human forearm. Am J Hypertens. 2000;13:1155–60.
Lee SJ, Levitsky K, Parlati F, Bennett MK, Arastu-Kapur S, Kellerman L, et al. Clinical activity of carfilzomib correlates with inhibition of multiple proteasome subunits: application of a novel pharmacodynamic assay. Br J Haematol. 2016;173:884–95.
Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–90.
Albornoz N, Bustamante H, Soza A, Burgos P. Cellular Responses to Proteasome Inhibition: Molecular Mechanisms and Beyond. Int J Mol Sci. 2019;20:3379.
Meiners S, Ludwig A, Lorenz M, Dreger H, Baumann G, Stangl V, et al. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic Biol Med. 2006;40:2232–41.
Kwon YT, Ciechanover A. The Ubiquitin code in the ubiquitin-proteasome system and autophagy. Trends Biochemical Sci. 2017;42:873–86.
Meiners S, Heyken D, Weller A, Ludwig A, Stangl K, Kloetzel PM, et al. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of Mammalian proteasomes. J Biol Chem. 2003;278:21517–25.
Tsakiri EN, Gumeni S, Vougas K, Pendin D, Papassideri I, Daga A, et al. Proteasome dysfunction induces excessive proteome instability and loss of mitostasis that can be mitigated by enhancing mitochondrial fusion or autophagy. Autophagy. 2019;15:1757–73.
Russell SD, Lyon A, Lenihan DJ, Moreau P, Joshua D, Chng W-J, et al. Serial echocardiographic assessment of patients (pts) with relapsed multiple myeloma (RMM) receiving carfilzomib and dexamethasone (Kd) vs bortezomib and dexamethasone (Vd): a substudy of the phase 3 Endeavor Trial (NCT01568866). Blood. 2015;126:4250.
Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, et al. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:7–17.
Charakida M, Masi S, Lüscher TF, Kastelein JJP, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. Eur Heart J. 2010;31:2854–61.
Moreau P, Mateos MV, Berenson JR, Weisel K, Lazzaro A, Song K, et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19:953–64.
Acknowledgements
We thank Mrs Marina Karakitsou for performing all vascular studies of this work.
Funding
AL has received funding from the Hellenic Foundation for Research and Innovation (HFRI) and the General Secretariat for Research and Technology (GSRT), under grant agreement No. [1285]. This study was also supported by institutional funding to EK, IPT, KS, and MAD.
Author information
Authors and Affiliations
Contributions
EK, K.Stamatelopoulos, IPT, and MAD designed research, analyzed, and interpreted data. AL, MG, NM, E-DP, EM, ET, and K.Stellos performed research. IPT and E-DP contributed vital new reagents or analytical tools and assayed proteasome activity. EE-P, DF, NK, ID, MK, MR, and MM collected data. GG and K.Stamatelopoulos performed statistical analysis. EK, K.Stamatelopoulos, IPT, AL, and GG wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
AMGEN financially supported part of the study as an investigator-initiated study (NCT03543579). EK reports honoraria from Amgen, Genesis Pharma, Takeda, Janssen and Prothena as well as research funding from Amgen and Janssen. KS received fees for being on the regional advisory board for Bayer. ET reports honoraria from Amgen, Celgene, Takeda, Novartis, Roche, Janssen, Bristol-Myers Squibb, and GlaxoSmithKline; consulting or advisory role at Takeda, Novartis, Janssen, Amgen, and Celgene; research funding from Amgen and Janssen; and travel, accommodations and expenses from Amgen, Janssen, Genesis Pharma, and Takeda. KS reports research funding and honoraria from Amgen. IPT reports research funding from Amgen. MAD reports honoraria from Amgen, Novartis, Celgene, Takeda, Janssen, and Bristol-Myers Squibb; consulting or advisory role at Amgen, Janssen, Takeda, and Celgene; research funding from Janssen and Amgen; and travel, accommodations, and expenses from Janssen. The rest of the authors report no conflicts.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kastritis, E., Laina, A., Georgiopoulos, G. et al. Carfilzomib-induced endothelial dysfunction, recovery of proteasome activity, and prediction of cardiovascular complications: a prospective study. Leukemia 35, 1418–1427 (2021). https://doi.org/10.1038/s41375-021-01141-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01141-4
This article is cited by
-
Endothelial function measured by peripheral arterial tonometry in patients with chronic myeloid leukemia on tyrosine kinase inhibitor therapy: a pilot study
Cardio-Oncology (2023)
-
An integrative review of nonobvious puzzles of cellular and molecular cardiooncology
Cellular & Molecular Biology Letters (2023)
-
Mutations in the alternative complement pathway in multiple myeloma patients with carfilzomib-induced thrombotic microangiopathy
Blood Cancer Journal (2023)
-
Endothelial dysfunction and thromboembolism in children, adolescents, and young adults with acute lymphoblastic leukemia
Leukemia (2022)