Abstract
The development of proteinuria restricts the dose of anti-angiogenic agents, thereby reducing their efficacy. Thus, this retrospective study was undertaken to identify predictive factors of the development of angiogenesis inhibitor-induced proteinuria, and to elucidate if there is a difference in the likelihood of proteinuria among anti-angiogenic agents or cancer types, to help guide future strategies to improve the safety, efficacy, and quality of life of patients receiving chemotherapy. Between April 2014 and February 2019, 124 cancer patients at our outpatient chemotherapy center who were receiving chemotherapy with bevacizumab, ramucirumab, or aflibercept were enrolled. Variables related to the development of proteinuria were extracted from the patients’ clinical records and used for regression analysis. The level of the proteinuria was evaluated based on CTCAE version 5. Multivariate ordered logistic regression analysis was performed to identify predictive factors for the development of proteinuria. The Wilcoxon/Kruskal-Wallis test was used to identify significant differences between groups. Significant factors identified included systolic blood pressure (SBP) [odds ratio (OR) = 1.031, 95% confidence interval (CI) = 1.005–1.058; P = 0.0197], number of cycles (OR = 1.049, 95% CI = 1.018–1.082; P = 0.0019), and calcium channel blocker use (OR = 2.589, 95% CI = 1.090–6.146; P = 0.0311). There was no difference among the three anti-angiogenic agents (P = 0.4969) or among cancer types (P = 0.2726) in the likelihood of proteinuria. In conclusion, SBP, number of cycles, and calcium channel blocker use were identified as significant predictors of the development of angiogenesis inhibitor-induced proteinuria. There was no difference among the three anti-angiogenic agents or among cancer types.
Similar content being viewed by others
Introduction
Anti-angiogenic agents targeting the human vascular endothelial growth factor pathway, such as bevacizumab, ramucirumab, and aflibercept, significantly improve overall survival and progression-free survival in colorectal, lung, gastric, breast cancers and so on1,2,3,4,5. The toxicity profile of anti-angiogenic agents differs from those of conventional cytotoxic anticancer agents; use of anti-angiogenic agents is commonly associated with elevated blood pressure, proteinuria, and bleeding1,2,3,4,5. The development of proteinuria restricts the dose of anti-angiogenic agents, thereby reducing their efficacy6,7. However, the predictive factors for proteinuria have not been fully elucidated, and few studies have examined the differences in the development of proteinuria among anti-angiogenic agents or cancer types.
Thus, this retrospective study was undertaken to identify predictive factors of the development of angiogenesis inhibitor-induced proteinuria and to elucidate if there is a difference in the likelihood of proteinuria among anti-angiogenic agents or cancer types to help guide future strategies to improve the safety, efficacy, and quality of life (QoL) of patients receiving chemotherapy.
Patients and Methods
Study period and participants
Between April 2014 and February 2019, this study enrolled 139 colon cancer, gastric cancer, breast cancer, and lung cancer patients who were receiving chemotherapy with anti-angiogenic agents at our outpatient chemotherapy center. The Medical Ethics Review Committee of the Kyoto Prefectural University of Medicine approved this study (Approval No. ERB-C-867-2). All procedures were performed in accordance with the ethical standards of the Medical Ethics Review Committee of the Kyoto Prefectural University of Medicine and the 1964 Helsinki Declaration and its later amendments. No prospective studies with human participants or animals were performed by any of the authors for this article. Due to the retrospective nature of this work, informed consent was waived for the individual participants included in the study in accordance with the ethical standards of the Medical Ethics Review Committee of the Kyoto Prefectural University of Medicine.
Extraction of variables
For the regression analysis of factors related to angiogenesis inhibitor-induced proteinuria, variables were extracted by manual abstraction from clinical records. Variables evaluated included factors that could potentially impact the development of proteinuria: demographic data (sex, age, height, weight, body mass index, and body surface area), Eastern Cooperative Oncology Group performance status, cancer type, presence of comorbidity (diabetes mellitus, hypertension), laboratory test values [albumin, serum creatinine, and estimated creatinine clearance, lactate dehydrogenase (LDH) and fibrinogen)], administration of anti-angiogenic agents (number of cycles, and doses of bevacizumab, ramucirumab, and aflibercept, respectively), medications that affect blood pressure [renin-angiotensin system (RAS) inhibitors, calcium channel blockers, and loop or thiazide diuretics], and vital signs [systolic blood pressure (SBP), diastolic blood pressure (DBP)]. The presence of hypertension was assessed by prescriptions for antihypertensive drugs. Creatinine clearance was estimated using the Cockcroft and Gault equation based on serum creatinine, sex, age, and weight. Clinical information was extracted before the first dose of bevacizumab, ramucirumab, or aflibercept. The level of angiogenesis inhibitor-induced proteinuria was evaluated based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
Explanatory variables were examined for multicollinearity (correlation coefficient |r| ≥ 0.7), since when correlations exist among the variables, this can lead to incorrect results of regression analyses.
Explanatory variables were selected based on correlation strength with the level of the angiogenesis inhibitor-induced proteinuria (objective variable) or clinical significance. First, univariate ordered logistic regression analysis between the outcomes and each potential explanatory variable was performed. Subsequently, a multivariate ordered logistic regression model was constructed by employing the stepwise selection procedure with the potential candidate variables. The model used a variable entry criterion of 0.25 and a variable retention criterion of 0.15. Ordered logistic regression analysis was employed, because the level of proteinuria was evaluated by a graded scale and multiple factors potentially involved as predictive factors for the development of proteinuria had to be evaluated simultaneously. Optimal cutoff thresholds were determined using receiver operating characteristic curve (ROC) analysis8. In many clinical trials, the equal or >2 proteinuria grade was the drug discontinuation criterion. Therefore, patients were divided in two groups depending the equal or >2 proteinuria grade or not. Furthermore, whether there was a difference in the development of proteinuria among these three anti-angiogenic agents or cancer types was analyzed. The Wilcoxon/Kruskal-Wallis test was used to identify significant differences between groups.
For all statistical analyses, values of P < 0.05 (2-tailed) were considered significant. All analyses were performed using JMP® version 14.3.0. (SAS Institute, Cary, NC, USA).
Results
Of the 139 patients who received chemotherapy with bevacizumab, ramucirumab, or aflibercept, 15 were excluded from this study: 3 were discontinued with only one course due to adverse events[1 patient had general fatigue, 2 patients had febrile neutropenia during chemotherapy (nab-paclitaxel plus ramucirumab and paclitaxel plus ramucirumab)] and 12 had insufficient data. Table 1 presents the clinical characteristics of the 124 enrolled patients, the potential variables related to the development of proteinuria, and the results of univariate analysis. Although fibrinogen was a significant predictor in univariate analysis, it was not used for multivariate analysis due to the large number of missing data (n = 84). Multicollinearity was observed in body height, body weight, body mass index (BMI) and body surface area (BSA). BSA, which was considered the most clinically relevant in cancer chemotherapy, was used for analysis. The forward stepwise selection procedure identified five variables (serum creatinine, SBP, number of cycles, calcium channel blockers, and RAS inhibitors).
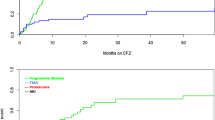
These variables were then used for multivariate ordered logistic regression analysis. Significant factors identified for the development of proteinuria included SBP [odds ratio (OR) = 1.031, 95% confidence interval (CI) = 1.005–1.058; P = 0.0197], number of cycles (OR = 1.049, 95% CI = 1.018–1.082; P = 0.0019), and calcium channel blockers (OR = 2.589, 95% CI = 1.090–6.146; P = 0.0311) (Table 2). The accuracy of the model was 82/124, expressed as the ratio of patients whose expected value was equal to the observed value. The ROC curve analysis of the group likely to develop proteinuria (≥Grade 2) showed that the threshold for the number of cycles was ≥13, with a sensitivity of 63.3% and specificity of 72.3% [area under the curve (AUC) = 0.66](Fig. 1A), and that of SBP was ≥135 mmHg, with a sensitivity of 48.3% and specificity of 79.8% [AUC = 0.64](Fig. 1B). ROC curve of calcium channel blockers showed with a sensitivity of 43.3% and specificity of 80.9% (AUC = 0.62) (Fig. 1C).
There was no difference in the development of proteinuria among the anti-angiogenic agents (bevacizumab, ramucirumab, and aflibercept; P = 0.4969), and there was also no difference among cancer types (colon, gastric, lung, and breast cancers; P = 0.2726).
Discussion
The multivariate ordered logistic regression analysis performed in this study showed that the significant predictors for the development of proteinuria included number of cycles, SBP (before the initial administration of anti-angiogenic agents), and calcium channel blockers. Fibrinogen was also a predictor, as determined by univariate analysis. On ROC curve analysis of the potential factors responsible for the development of proteinuria, the cut-off value for the number of cycles was ≥13, and that of SBP was ≥135 mmHg. This study also showed that the likelihood of proteinuria was not different among anti-angiogenic agents or cancer types.
Several studies have reported that the development of angiogenesis inhibitor-induced proteinuria is dose-dependent9,10,11,12. The result of the current study is consistent with this previous finding. Thus, clinicians need to know that the incidence and severity of proteinuria increase as the number of administration cycles of anti-angiogenic agents increase, especially in patients with ≥13 cycles.
In the current study, the SBP cut-off value for the development of proteinuria was ≥135 mmHg. Previous studies demonstrated that high blood pressure is a major risk factor for proteinuria in the general population13. It has also been shown that SBP ≥130 mmHg is a risk factor for bevacizumab-induced proteinuria14; similarly, the present results showed that SBP ≥135 mmHg was a risk factor for proteinuria. Clinicians need to pay attention to blood pressure control before treatment.
Furthermore, the present study found that calcium channel blocker use is a risk factor for proteinuria. On the other hand, RAS inhibitor use was neither a protective factor nor a risk factor. As in previous research, the present results suggest that RAS inhibitor administration reduces the risk of proteinuria15,16,17,18. During treatment with anti-angiogenic agents, RAS inhibitors may be recommended for hypertension. Further research is needed on this issue.
Previous studies suggested that angiogenesis inhibitor-induced proteinuria is more likely to develop with colorectal cancer15,19. However, in the present study, there was no difference in the likelihood of proteinuria among cancer types. On the other hand, univariate analysis showed that proteinuria was more likely to occur in patients with colorectal cancer, but it was not significant on multivariate analysis. In patients with colorectal cancer, special attention may be needed regarding the development of proteinuria, but further study of this issue is needed. Fibrinogen was also a predictor, as determined by univariate analysis. This was consistent with previous studies20. Clinicians also need to pay attention elevated fibrinogen levels.
In the present study, there was no difference in the likelihood of proteinuria depending on anti-angiogenic agents. A previous study suggested that severe renal side effects may be less common with ramucirumab than with bevacizumab21. On the other hand, Peng et al. showed that the risk of developing all-grade and high-grade proteinuria was substantially higher with aflibercept than with bevacizumab7.
There were several limitations to the current study. First, the retrospective design of the research may have decreased the reliability of the data extracted. Second, since this study was conducted at a single institute, it only analyzed a relatively small number of patients. Therefore, prospective multicenter study will be needed to confirm these results.
In conclusion, SBP, number of cycles, and concomitant use of calcium channel blockers were identified as significant predictors of the development of proteinuria in cancer patients treated with bevacizumab, ramucirumab, or aflibercept. In addition, it was found that the likelihood of proteinuria was not different among anti-angiogenic agents or cancer types. However, these preliminary findings will need to be confirmed in a larger randomized controlled trial. There is a need to identify cancer patients who could develop side effects, in order to be able, in the future, to select them or/and treat them with more adequate doses of anti-angiogenic agents. Nevertheless, these findings may assist in developing chemotherapeutic strategies that can be used to improve the safety and efficacy including anti-angiogenic agents, as well as the QoL of patients.
ROC curves about proteinuria (≥Grade 2) according the logistic regression significant variables. (A) ROC curve of the number of cycles with a sensitivity of 63.3% and specificity of 72.3% (AUC = 0.66). (B) ROC curve of systolic blood pressure with a sensitivity of 48.3% and specificity of 79.8% (AUC = 0.64). (C) ROC curve of calcium channel blockers with a sensitivity of 43.3% and specificity of 80.9% (AUC = 0.62).
References
Kabbinavar, F. F. et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J. Clin. Oncol. 23, 3706–12 (2005).
Sandler, A. et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 355, 2542–50 (2006).
Wilke, H. et al. RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 15, 1224–35 (2014).
von Minckwitz, G. et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. 15, 1269–78 (2014).
Fernández Montes, A. et al. Juez Martel, López Flores M, Carmona-Bayonas A. Prognostic Nomogram and Patterns of Use of FOLFIRI-Aflibercept in Advanced Colorectal Cancer: A Real-World Data Analysis. Oncologist. 24, e687–e695 (2019).
Abdel-Rahman, O. & ElHalawani, H. Proteinuria in Patients with Solid Tumors Treated with Ramucirumab: A Systematic Review and Meta-Analysis. Chemotherapy. 60, 325–33 (2014).
Peng, L. et al. Incidence and risk of proteinuria with aflibercept in cancer patients: a meta-analysis. PLoS One. 9, e111839 (2014).
Akobeng, A. K. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 96, 644–7 (2007).
Wu, S., Kim, C., Baer, L. & Zhu, X. Bevacizumab increases risk for severe proteinuria in cancer patients. J. Am. Soc. Nephrol. 21, 1381–9 (2010).
Lafayette, R. A. et al. Incidence and relevance of proteinuria in bevacizumab-treated patients: pooled analysis from randomized controlled trials. Am. J. Nephrol. 40, 75–83 (2014).
Zhu, X., Wu, S., Dahut, W. L. & Parikh, C. R. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am. J. Kidney Dis. 49, 186–93 (2007).
Fujii, T., Kawasoe, K., Tonooka, A., Ohta, A. & Nitta, K. Nephrotic syndrome associated with ramucirumab therapy: A single-center case series and literature review. Med. 98, e16236 (2019).
George, B. A., Zhou, X. J. & Toto, R. Nephrotic syndrome after bevacizumab: case report and literature review. Am. J. Kidney Dis. 49, e23–9 (2007).
Teramachi, H. et al. Risk factors contributing to urinary protein expression resulting from bevacizumab combination chemotherapy. Pharmazie. 68, 217–20 (2013).
Hirai, T., Shuji, Y., Takiyama, M., Hanada, K. & Itoh, T. Renin-angiotensin system inhibitors for countering proteinuria induced by angiogenesis inhibitors: a retrospective observational analysis. Cancer Chemother. Pharmacol. 84, 195–202 (2019).
Vogt, L., Waanders, F., Boomsma, F., de Zeeuw, D. & Navis, G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J. Am. Soc. Nephrol. 19, 999–1007 (2008).
Lambers Heerspink, H. J., de Borst, M. H., Bakker, S. J. & Navis, G. J. Improving the efficacy of RAAS blockade in patients with chronic kidney disease. Nat. Rev. Nephrol. 9, 112–21 (2013).
Nihei, S. et al. Antiproteinuric effects of renin-angiotensin inhibitors in lung cancer patients receiving bevacizumab. Cancer Chemother. Pharmacol. 81, 1051–1059 (2018).
Nakaya, A. et al. Retrospective analysis of bevacizumab-induced hypertension and clinical outcome in patients with colorectal cancer and lung cancer. Cancer Med. 5, 1381–7 (2016).
Muntner, P., He, J., Astor, B. C., Folsom, A. R. & Coresh, J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 16, 529–38 (2005).
Arnold, D. et al. Meta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumab. Ann. Oncol. 28, 2932–2942 (2017).
Author information
Authors and Affiliations
Contributions
Yuko Kanbayashi: concept and design, data acquisition, data analysis, data interpretation, manuscript writing, and final approval of the manuscript. Takeshi Ishikawa: concept and design, data acquisition, data interpretation, manuscript writing, and final approval of the manuscript. Yusuke Tabuchi: concept and design, manuscript writing, and final approval of the manuscript. Koichi Sakaguchi: concept and design, data acquisition, manuscript writing, and final approval of the manuscript. Yoshimi Ouchi: data acquisition, manuscript writing, and final approval of the manuscript. Eigo Otsuji: concept and design, data acquisition, manuscript writing, and final approval of the manuscript. Koichi Takayama concept and design, data acquisition, manuscript writing, supervision and final approval of the manuscript. Tetsuya Taguchi: concept and design, data acquisition, manuscript writing, supervision and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanbayashi, Y., Ishikawa, T., Tabuchi, Y. et al. Predictive factors for the development of proteinuria in cancer patients treated with bevacizumab, ramucirumab, and aflibercept: a single-institution retrospective analysis. Sci Rep 10, 2011 (2020). https://doi.org/10.1038/s41598-020-58994-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58994-5
This article is cited by
-
Impact of preexisting proteinuria on the development of regorafenib-induced problematic proteinuria in real-world metastatic colorectal cancer treatment
Scientific Reports (2024)
-
Machine learning application identifies plasma markers for proteinuria in metastatic colorectal cancer patients treated with Bevacizumab
Cancer Chemotherapy and Pharmacology (2024)
-
Updates on the mechanisms of toxicities associated with monoclonal antibodies targeting growth factor signaling and immune cells in cancer
Toxicological Research (2024)
-
Evaluation of lung toxicity with bevacizumab using the spontaneous reporting database
Scientific Reports (2022)
-
Risk factors of proteinuria and potentially protective effect of renin–angiotensin system inhibitors in patients with renal cell carcinoma receiving axitinib
Cancer Chemotherapy and Pharmacology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.