Abstract
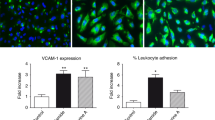
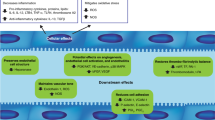
Multiple myeloma induction treatment includes proteasome inhibitors (PI) and immunomodulatory agents at present. The incidence of engraftment syndrome, a transplant complication potentially related to endothelium, has increased in the last years. Our aim was to investigate whether bortezomib (Velcade, V), thalidomide (T), and dexamethasone (D) affect the endothelium, and explore defibrotide (DF) as protective agent. Endothelial cells (ECs) in culture were exposed to the compounds separately or in combination, without (VTD) and with DF (VTD + DF). Changes in markers of: (i) inflammation (ICAM-1 expression and leukocyte adhesion), (ii) VWF production, (iii) cell permeability (VE-cadherin expression and cell monolayer integrity), and (iv) oxidative stress (ROS production and eNOS expression) were measured. ICAM-1 and VWF expression increased significantly in VTD but were similar to controls in VTD + DF. Separately, bortezomib was the main deleterious agent whereas dexamethasone showed no harmful effect. Leukocyte adhesion showed similar trends. VE-cadherin expression was lower in VTD and normalized in VTD + DF. EC permeability increased only with bortezomib. No changes were observed in oxidative stress markers. Our results demonstrate that bortezomib damages the endothelium, and DF prevents this effect. A better knowledge of the induction drugs impact will allow the design of measures to protect the endothelium.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Li Z, Rubinstein SM, Thota R, Savani M, Brissot E, Shaw BE, et al. Immune-mediated complications after hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2016;22:1368–75.
Koniarczyk HL, Ferraro C, Miceli T. Hematopoietic stem cell transplantation for multiple myeloma. Semin Oncol Nurs. 2017;33:265–78.
Gerecke C, Fuhrmann S, Strifler S, Schmidt-Hieber M, Einsele H, Knop S. Medicine: the diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. 2016;113:470–6.
Cavo M, Terpos E, Bargay J, Einsele H, Cavet J, Greil R, et al. The multiple myeloma treatment landscape: international guideline recommendations and clinical practice in Europe. Expert Rev Hematol. 2018;11:219–37.
Cornell RF, Hari P, Zhang M-J, Zhong X, Thompson J, Fenske TS, et al. Divergent effects of novel immunomodulatory agents and cyclophosphamide on the risk of engraftment syndrome after autologous peripheral blood stem cell transplantation for multiple myeloma. Biol Blood Marrow Transpl. 2013;19:1368–73.
Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transpl. 2011;46:1495–502.
DeLeve LD, Wang X, Kanel GC, Ito Y, Bethea NW, McCuskey MK, et al. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology. 2003;38:900–8.
DeLeve LD, Wang X, Tsai J, Kanel G, Strasberg S, Tokes ZA. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology. 2003;125:882–90.
DeLeve LD, Ito Y, Bethea NW, McCuskey MK, Wang X, McCuskey RS. Embolization by sinusoidal lining cells obstructs the microcirculation in rat sinusoidal obstruction syndrome. Am J Physiol Liver Physiol. 2003;284:G1045–52.
Nürnberger W, Willers R, Burdach S, Göbel U. Risk factors for capillary leakage syndrome after bone marrow transplantation. Ann Hematol. 1997;74:221–4.
Afessa B, Tefferi A, Litzow MR, Krowka MJ, Wylam ME, Peters SG. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166:641–5.
George JN. ADAMTS13, thrombotic thrombocytopenic purpura, and hemolytic uremic syndrome. Curr Hematol Rep. 2005;4:167–9.
George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion. 2004;44:294–304.
Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martine C, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biol Blood Marrow Transplant. 2010;16:985–93.
Maiolino A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M. Post-transplant complications engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria. Bone Marrow Transplant. 2003;31:393–7.
Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–8.
Katzel JA, Mazumder A, Jagannath S, Vesole DH. Engraftment syndrome after hematopoietic stem cell transplantation in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:151.
Gao Y, Ma G, Liu S, Teng Y, Wang Y, Su Y. Thalidomide and multiple myeloma serum synergistically induce a hemostatic imbalance in endothelial cells in vitro. Thromb Res. 2015;135:1154–9.
Na W, Shin JY, Lee JY, Jeong S, Kim WS, Yune TY, et al. Dexamethasone suppresses JMJD3 gene activation via a putative negative glucocorticoid response element and maintains integrity of tight junctions in brain microvascular endothelial cells. J Cereb Blood Flow Metab. 2017;37:3695–708.
Palomo M, Diaz-Ricart M, Carreras E. Endothelial dysfunction in hematopoietic cell transplantation. Clin Hematol Int. 2019;1:45.
Akil A, Zhang Q, Mumaw CL, Raiker N, Yu J, Velez de Mendizabal N, et al. Biomarkers for diagnosis and prognosis of sinusoidal obstruction syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:1739–45.
Mir E, Palomo M, Rovira M, Pereira A, Escolar G, Penack O, et al. Endothelial damage is aggravated in acute GvHD and could predict its development. Bone Marrow Transplant. 2017;52:1317–25.
Martinez-Sanchez J, Hamelmann H, Palomo M, Mir E, Moreno-Castaño AB, Torramade S, et al. Acute graft-vs.-host disease-associated endothelial activation in vitro is prevented by defibrotide. Front Immunol. 2019;10:2339.
Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Escolar G, et al. The release of soluble factors contributing to endothelial activation and damage after hematopoietic stem cell transplantation is not limited to the allogeneic setting and involves several pathogenic mechanisms. Biol Blood Marrow Transplant. 2009;15:537–46.
Gutiérrez-García G, Rovira M, Magnano L, Rosiñol L, Bataller A, Suárez-Lledó M, et al. Innovative strategies minimize engraftment syndrome in multiple myeloma patients with novel induction therapy following autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2018;53:1541–7.
Eissner G, Multhoff G, Gerbitz A, Kirchner S, Bauer S, Haffner S, et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood. 2002;100:334–40.
Palomo M, Vera M, Martin S, Torramadé-Moix S, Martinez-Sanchez J, Moreno AB, et al. Up-regulation of HDACs, a harbinger of uraemic endothelial dysfunction, is prevented by defibrotide. J Cell Mol Med. 2019;24:1713–23.
Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16:1005–17.
Rosiñol L, Oriol A, Teruel AI, Hernández D, López-Jiménez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–96.
Carmona A, Díaz-Ricart M, Palomo M, Molina P, Pino M, Rovira M, et al. Distinct deleterious effects of cyclosporine and tacrolimus and combined tacrolimus–sirolimus on endothelial cells: protective effect of defibrotide. Biol Blood Marrow Transplant. 2013;19:1439–45.
Malard F, Harousseau JL, Mohty M. Anti-tumour treatment multiple myeloma treatment at relapse after autologous stem cell transplantation: a practical analysis. Cancer Treat Rev. 2017;52:41–7.
Oyama Y, Cohen B, Traynor A, Brush M, Rodriguez J, Burt RK. Case report engraftment syndrome: a common cause for rash and fever following autologous hematopoietic stem cell transplantation for multiple sclerosis. Bone Marrow Transplant. 2002;29:81–5.
Carreras E, Fernádez-Avilés F, Silva L, Guerrero M, Fernández De Larrea C, Martínez C, et al. Engraftment syndrome after auto-SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone Marrow Transplant. 2010;45:1417–22.
Falci SGM, Lima TC, Martins CC, Dos Santos CRR, Pinheiro MLP. Preemptive effect of dexamethasone in third-molar surgery: A Meta-analysis. Anesth Prog. 2017;64:136–43.
Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36:561–84.
Kubiczkova L, Pour L, Sedlarikova L, Hajek R, Sevcikova S. Proteasome inhibitors—molecular basis and current perspectives in multiple myeloma. J Cell Mol Med. 2014;18:947–61.
Ludwig A, Fechner M, Wilck N, Meiners S, Grimbo N, Baumann G, et al. Potent anti-inflammatory effects of low-dose proteasome inhibition in the vascular system. J Mol Med. 2009;87:793–802.
Vestweber D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–32.
Li W, Fu J, Zhang S, Zhao J, Xie N, Cai G. The proteasome inhibitor bortezomib induces testicular toxicity by upregulation of oxidative stress, AMP-activated protein kinase (AMPK) activation and deregulation of germ cell development in adult murine testis. Toxicol Appl Pharm. 2015;285:98–109.
Sahni A, Thomasson ED, Shah R, Sahni SK. Bortezomib effects on human microvascular endothelium in vitro. Pharmacology. 2016;98:272–8.
Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013;54:683–7.
Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med. 2001;344:1951–2.
Rafiee P, Stein DJ, Nelson VM, Otterson MF, Shaker R, Binion DG. Thalidomide inhibits inflammatory and angiogenic activation of human intestinal microvascular endothelial cells (HIMEC). Am J Physiol Gastrointest Liver Physiol. 2010;298:G167–76.
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23.
Swan D, Rocci A, Bradbury C, Thachil J. Venous thromboembolism in multiple myeloma—choice of prophylaxis, role of direct oral anticoagulants and special considerations. Br J Haematol. 2018;183:538–56.
Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME. Defibrotide: properties and clinical use of an old/new drug. Vasc Pharm. 2013;59:1–10.
Palomo M, Diaz-Ricart M, Rovira M, Escolar G, Carreras E. Defibrotide prevents the activation of macrovascular and microvascular endothelia caused by soluble factors released to blood by autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:497–506.
Palomo M, Mir E, Rovira M, Escolar G, Carreras E, Diaz-Ricart M. What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance. Blood. 2016;127:1719–27.
Acknowledgements
We thank the Primary Hemostasis Laboratory staff (Hospital Clinic, Barcelona) for technical support, and Dr Urooj Zafar for his constructive comments.
Funding
Study partially supported by Jazz Pharmaceuticals (IST-16-10355); German José Carreras Leukaemia Foundation (Grant 03 R/2019); Instituto de Salud Carlos III, Spanish Government (PIE15/00027, DTS16/00133, and FIS PI19/00888), and Generalitat de Catalunya (2017-SGR675, CERCA).
Author information
Authors and Affiliations
Contributions
JM-S designed and conducted the experiments, analyzed the results, designed the images, and wrote the manuscript. MP contributed to the design and conducted the experiments, analyzed the results, and reviewed the manuscript. ST-M participated in the experiments, cell culture maintenance, and figures. ABM-C, MR, GG-G, FF-A, GE, OP, and LR reviewed results and manuscript. EC and MD-R designed, supervised, and reviewed the study, results, and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Research partially supported by Jazz Pharmaceuticals plc/Gentium Inc. MP received speaker’s fees from Jazz Pharmaceuticals. GG-G received a grant from Pfizer and Gilead. GE, OP, EC, and MD-R received research funding and speaker’s fees from Jazz Pharmaceuticals. LR received honoraria from Janssen, Celgene, Amgen, and Takeda. The other authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martinez-Sanchez, J., Palomo, M., Torramade-Moix, S. et al. The induction strategies administered in the treatment of multiple myeloma exhibit a deleterious effect on the endothelium. Bone Marrow Transplant 55, 2270–2278 (2020). https://doi.org/10.1038/s41409-020-0947-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0947-9
This article is cited by
-
Mafosfamide, a cyclophosphamide analog, causes a proinflammatory response and increased permeability on endothelial cells in vitro
Bone Marrow Transplantation (2023)
-
An endothelial proinflammatory phenotype precedes the development of the engraftment syndrome after autologous Hct
Bone Marrow Transplantation (2022)