Abstract
Peritoneal fibrosis is a common complication of peritoneal dialysis (PD) with a complicated pathogenesis and limited treatments. Parthenolide (PTL), a recognized nuclear factor-κB (NF-κB) inhibitor extracted from Tanacetum balsamita, has been widely used to treat various inflammatory diseases and has been proven to improve peritoneal fibrosis in PD mice by selectively inhibiting the phosphorylation of Smad2/3. Transforming growth factor-β1 (TGF-β1), via Smad-dependent signaling, has a pivotal role in promoting pathogenic of fibrosis. To investigate whether PTL can inhibit peritoneal fibrosis, we affected the interaction between NF-κB and the TGF-β/Smad2/3 pathway. Long dwell peritoneal dialysis fluid (PDF) and peritoneum tissues were collected from continuous ambulatory peritoneal dialysis (CAPD) patients. PTL was administered intragastrically into a PD mouse model by daily infusion of 4.25% dextrose-containing PDF. Treated HMrSV5 cells or rat peritoneal mesothelial cells (RPMCs) were treated with high glucose(138 mM) at the same concentration as 2.5% dextrose-containing PDF and PTL. PD-related peritoneal fibrosis samples indicated an increase in inflammation, and PTL decreased the levels of inflammatory cytokines (L-6, TNF-α, and MCP-1). PTL inhibited high glucose-induced mesothelial-to-mesenchymal transition (MMT), as indicated by a reduced expression of fibrosis markers (fibronectin, collagen I, and α-SMA) and increased expression of the epithelial marker E-cadherin. PTL also significantly decreased TGF-β1 expression and the phosphorylation of IκBα and NF-κBp65. The changes in the levels of TGF-β1 expression and p-p65 or p65 showed similar trends according to western blot, immunohistochemistry, and immunofluorescence assays in vitro and in vivo. Chromatin immunoprecipitation (ChIP) and luciferase reporter assays were used to confirm that PTL regulates the transcription of TGF-β1 induced by high glucose through NF-κBp65. In summary, PTL induces a therapeutic effect in peritoneal fibrosis by inhibiting inflammation via the NF-κB/ TGF-β/Smad signaling axis.
Similar content being viewed by others
Introduction
The prevalence and mortality of chronic kidney disease (CKD) are increasing, and CKD has become an important public health issue1,2. Approximately 2% of the patients develop end-stage renal disease (ESRD) every year and require renal replacement treatment3. Peritoneal dialysis (PD) is a safe, effective, convenient, and home-based renal replacement therapy4,5. More than 272,000 ESRD patients have received PD treatment, accounting for approximately 11% of dialysis patients worldwide6. However, with the prolongation of dialysis time, biologically incompatible factors such as high sugar levels, low pH, glucose degradation products in the peritoneal dialysis fluid (PDF), and recurrent peritonitis can cause peritoneal fibrosis. This leads to ultrafiltration failure and eventually to increased mortality of PD patients7,8. Peritoneal fibrosis is widespread, and its pathogenesis is complex, but there is currently no clear and effective treatment.
Mesothelial-to-mesenchymal transition (MMT) of peritoneal mesothelial cells is an early and reversible step of peritoneal fibrosis that plays an important role in the occurrence and progression of PD-related peritoneal fibrosis9,10. Transforming growth factor-β1 (TGF-β1)-induced peritoneal MMT is a key process of progressive peritoneal fibrosis11. Parthenolide (PTL) is a sesquiterpene lactone compound extracted from Tanacetum balsamita. It has mild effects, few adverse reactions, and has been widely used to treat various inflammatory conditions, including fever, migraines, and arthritis12,13,14,15.
Our previous paper revealed that PTL can effectively inhibit peritoneal fibrosis in a mouse peritoneal fibrosis model and MMT induced by HMrSV5 cells via TGF-β1. PTL selectively inhibits the phosphorylation of Smad2/3 but does not affect the phosphorylation of Smad1/5/9, p38, ERK, or AKT16. Selective blockade of the TGF-β/Smad2/3 signaling pathway may be a mechanism by which PTL improves peritoneal fibrosis. However, PTL is a recognized NF-κB inhibitor17,18, and there is crosstalk between the NF-κB and TGF-β signaling pathways in many cell types19. Whether PTL can improve peritoneal fibrosis by inhibiting inflammation or the interaction between NF-κBp65 and TGF-β1 needs further investigation.
In this study, we highlight that PTL can inhibit the inflammatory state of PD-associated peritoneal fibrosis in vivo and in vitro. This will confirm that PTL inhibits NF-κB targeting of the TGF-β1 promoter, thus inhibiting the effect of the TGF-β/Smad2/3 signaling pathway on PD-related peritoneal fibrosis.
Materials and Methods
MTT assay
In line with our previously reported method16, the MTT assay was used to evaluate the toxicity of HMrSV5 cells to glucose. HMrSV5 cells were treated with different concentrations of glucose for 48 h. Cell viability was tested by adding 20 μL of MTT (5 mg/ml) per well for 4 h and subcultured in a medium with 150 μL of dimethyl sulfoxide (DMSO; Sigma–Aldrich, Missouri, USA). The absorbance was measured at 490 nm.
PDF and peritoneal tissues from continuous ambulatory peritoneal dialysis (CAPD) patients
The long dwell PDF (from 10:00 pm to 8:00 am) was obtained from CAPD patients, including 15 patients who received PD for at least 1 month but less than 3 years (control group) and 15 patients who received PD for more than 5 years (PF group). After the PDF was evenly mixed, 10 mL was aseptically extracted and stored at −80 °C for testing. Anterior peritoneal tissues that adhered to the tube were obtained from CAPD patients who had PD for more than 5 years but stopped receiving PD because of peritoneal dysfunction. All patients used a conventional glucose-based PD solution and did not have a history of peritonitis within the previous 3 months, abdominal surgery, malignant tumor, or long-term use of immunosuppressants.
In addition, the discarded hernia sac and attached visceral peritoneum samples from traditional herniorrhaphy were used as healthy controls. The study was approved by the Clinical Research and Application Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University (2020-LCYJ-ZF-21).
Animal experiments
The experimental animal protocol was approved by the Institutional Animal Care and Use Committee of The Second Affiliated Hospital of Guangzhou Medical University in Guangzhou, China (No. A2019-021). C57BL/6J mice (body weight 20–22 g) were purchased from Pengyue Experimental Animal Breeding Co., Ltd (Jinan, China). PTL was provided by Accendatech Co., Ltd (Tianjin, China). Animal experiments were performed according to our previously reported method16. A peritoneal fibrosis mouse model was established by a daily intraperitoneal injection of 3 mL 4.25% glucose dialysis solution (Baxter HealthCare, Deerfield, IL, USA) for 28 days. For PTL treatment, mice received intragastric administration of PTL (12.5 mg/kg, 25 mg/kg and 50 mg/kg) following daily PDF infusion. PTL concentrations were 1.25 mg/mL, 2.5 mg/mL and 5 mg/mL, and mice were given 0.1 mL/10 g/d. PD mice treated with vehicle (normal saline) were also sacrificed at day 28 to serve as the untreated group. For control, mice received daily intraperitoneal injection of saline (3 mL) and treated with saine (0.1 ml/10 g/d) by intragastric administration for 28 days. Experimental groups were presented in Supplementary Table S1. Peritoneal tissues, except the injection site, were collected. Parietal peritoneum samples were fixed in 4% paraformaldehyde and visceral peritoneum samples were stored at −80 °C.
Rat peritoneal mesothelial cells (RPMCs) isolation
The isolation and culture of RPMCs have been described previously20. A male Sprague-Dawley rat weighing 180–200 g was purchased from Zhuhai Bes Test Bio-Tech Co., Ltd (Zhuhai, China). First, 30 mL of 0.25% trypsin-0.02% EDTA (Gibco, Massachusetts, USA) was infused into the rat abdominal cavity. The fluid was removed from the peritoneal cavity under sterile conditions after 20 min. To harvest RPMCs, cellular components were isolated by centrifugation, washed with PBS, and suspended in DMEM/F12 medium (Gibco, Massachusetts, USA) supplemented with 5% ITS (Sigma–Aldrich, Missouri, USA). The suspension was evenly inoculated into two 25 cm2 culture flasks and placed in an incubator at 37 °C with 5% CO2 for static culture. Cells were isolated based on differential time attachment after 1 h, and morphology was observed under a microscope after 48 h.
Cell culture and treatment
The human peritoneal mesothelial cell line HMrSV5 (kindly provided by Professor Xueqing Yu, Guangdong Provincial People’s Hospital, Guangzhou, China) was cultured in DMEM/F-12 medium (Gibco, Massachusetts, USA) supplemented with 10% fetal bovine serum (Gibco, Massachusetts, USA). RPMCs and HMrSV5 cells were maintained in an incubator containing 5% CO2 at 37 °C. In this experiment, cells were exposed to glucose (Macklin, Shanghai, China) or mannitol (Macklin, Shanghai, China) for 24 h and 48 h in the presence or absence of different concentrations of PTL. The PTL used in the cellular experiments was dissolved in DMSO (Sigma–Aldrich, Missouri, USA).
Enzyme-linked immunosorbent assay (ELISA)
Levels of inflammatory cytokines in PDF were measured using ELISA kits specific for human IL-6, TNF-α, and MCP-1 (MEIMIAN, Jiangsu, China) according to the manufacturer’s instructions. The sensitivities and detection ranges of the human IL-6, TNF-α, MCP-1 kits were 0.1, 0.1, 1 pg/mL and 1.5–48, 2.5–80, 10–320 pg/mL, respectively.
Quantitative real-time (qRT–PCR) analysis
The procedure for qRT–PCR has been described previously21. Total RNA was prepared from omentum tissues and HMrSV5 cells using TRIzol reagent (AG Accurate Biology, Changsha, China). Target gene expression was tested with SYBR Green Pro Taq HS (AG Accurate Biology, Changsha, China). The primer sequences included mouse IL-6(5′-CCGCTATGAAGTTCCTCTC-3′ and 3′-GGTATCCTCTGTGAAGTCTC-5′), mouse MCP-1(5′- TGCCCTAAGGTCTTCAGCAC-3′ and 3′- AAGGCATCACAGTCCGAGTC-5′), human IL-6(5′-CACTGGTCTTTTGGAGTTTGAG-3′ and 3′-GGACTTTTGTACTCATCTGCAC-5′), human TNF-α (5′-TGTAGCCCATGTTGTAGCAAACC-3′and 3′-GAGGACCTGGGAGTGATGAGGTA-5′), human MCP-1 (5′-GAGGCTGAGACTAACCCAGAAACATC-3′and 3′-GGGAATGAAGGTGGCTGCTATGAG-5′), mouse β-actin (B661302, Sangon Biotech, Shanghai, China), human β-actin (B661102, Sangon Biotech, Shanghai, China).
Western blot analysis
Visceral peritoneum samples, whole-cell lysates of HMrSV5 cells, and RPMCs were prepared using RIPA lysis buffer containing PMSF (Sigma–Aldrich, Missouri, USA) and phosphatase inhibitor (Beyotime, Shanghai, China). Protein concentrations were measured by a protein assay BCA kit (Thermo Fisher Scientific, Massachusetts, USA). Samples were analyzed by western blot analysis as described previously22. The primary antibodies included anti-collagen I (Boster, Wuhan, China), anti-fibronectin, anti- TGF-β1, anti-p65 (Abcam, Cambridge, Britain), anti-E-cadherin (BD Biosciences, USA), anti-α-SMA (Sigma–Aldrich, Missouri, USA), anti-p-p65, anti-IκBα, anti-p-IκBα (Cell Signaling Technology, Massachusetts, USA), anti-GAPDH (Rayantibody, Beijing, China), and anti-β-actin (EarthOx Life Science, California, USA) antibodies.
Immunofluorescence
Immunofluorescence staining was performed to identify RPMCs. To examine the relationship of p65 with TGF-β1 expression, two–color immunofluorescence was performed on HMrSV5 cells. Cell slides were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100, and blocked with 5% bovine serum albumin (Beyotime, Shanghai, China). The slides were incubated with anti-α-SMA (Sigma–Aldrich, Missouri, USA), anti-CK18, anti-vimentin, anti-TGF-β1(Servicebio, Wuhan, China) and anti-p65 (Abcam, Cambridge, Britain) antibodies overnight at 4 °C, followed by incubation with Alexa 488/555-conjugated secondary antibodies (ab176753/ab150106; Abcam). Nuclei were stained with DAPI (Sigma–Aldrich, Missouri, USA) for 10 min at room temperature. Images were taken by a fluorescence microscope (Ti-E-Nikon, Tokyo, Japan).
Histology and immunohistochemistry
Parietal peritoneum samples from patients and mice were cut into 4-μm-thick sections and prepared by a routine procedure. Masson’s trichrome staining and immunohistochemical staining were performed as described previously16. The primary antibodies used were anti-p65 (Cell Signaling Technology, Massachusetts, USA) and anti-TGF-β1(Abcam, Cambridge, Britain) antibodies.
Chromatin immunoprecipitation (ChIP)
ChIP was performed to verify the binding of the protein NF-κBp65 (RELA) to the promoter region of the TGF-β1 gene. A PierceTM agarose ChIP kit (26156; Thermo Fisher Scientific) was used for ChIP. HMrSV5 cells were treated with glucose (138 mM) or glucose (138 mM) + PTL (5 μM). Following the manufacturer’s protocol, cells were exposed to 1% formaldehyde at room temperature for 10 min to induce DNA cross-linking and then lysed and digested with MNase for 10 min on ice. The cell lysate supernatant was incubated with IgG, anti-RNA polymerase IIantibody, or p65 antibody (218533; Abcam) at 4 °C overnight and then with ChIP–Grade Protein A/G Plus Agarose for 1 h. The DNA-protein-antibody complexes were pulled down with magnetic beads and collected. DNA-protein crosslinking was removed by NaCl and protease K treatment. DNA was recovered by a DNA purification column and further analyzed by RT–PCR.
The primer pairs were as follows: TGF-β1-QPCR-1F: CCTCGGCGACTCCTTCC, TGF-β1-QPCR-1R: GCCATCTCCCTCCCACCT; TGF-β1-QPCR-2F: AAGCGCCACCAAAGCG, TGF-β1-QPCR-2R: ACTCTCCTTCCGTTCTGGGTC; TGF-β1-QPCR-3F: GGAAGCATCTTTCTCTCCTTCCA, TGF-β1-QPCR-3R: CAGAGTCTAGCACAGGAGAAGGG; GAPDH-QPCR-F: CATGGGTGTGAACCATGAGA, GAPDH-QPCR-R: GTCTTCTGGGTGGCAGTGAT.
Luciferase reporter assay
A luciferase reporter assay was performed according to a standard protocol. The promoter region of TGF-β1 was cloned into the PGL3 vector, and the p65 coding sequence (CDS) region was cloned into the pcDNA3.1 overexpression vector. The positive clones were selected to extract plasmids for DNA sequencing, and a plasmid kit (DP107-02; TIANGEN, Beijing, China) was used to extract enough plasmids for later use. To verify the interaction between the p65 protein and the regulatory region of the TGFβ1 promoter, the following experimental groups were created: pRL-TK + pcDNA3.1-NC + PGL3-NC; pRL-TK + pcDNA3.1-NC + PGL3 -TGFβ1; pRL-TK + pcDNA3.1-p65 + PGL3-TGFβ1; pRL-TK + PTL (5 μM) + PGL3-TGFβ1; pRL-TK + PTL (5 μM) + pcDNA3.1-p65 + PGL3-TGFβ1. The indicated plasmids and pRL-TK Renilla plasmid were cotransfected into HMrSV5 cells using Lipofectamine 2000 Reagent (Invitrogen, Thermo Fisher Scientific). After transfection for 48 h, luciferase and Renilla signals were determined.
Statistical analyses
Data are expressed as the mean ± SD. Comparisons between two groups were made using Student’s t-test. Comparisons among multiple groups were performed using one-way ANOVA followed by either a least significant difference (LSD) test or Dunnett’s T3 test, depending on the results of a homogeneity of variance test. Statistical analyses were conducted with SPSS 22.0 (IBM, New York, USA). P < 0.05 was considered statistically significant.
Results
The toxic effects of glucose on HMrSV5 cells
First, an MTT assay of HMrSV5 cells stimulated with glucose (0, 83, 138, and 236 mM) at the same concentration as conventional glucose-based PD solution (0, 1.5%, 2.5%, and 4.25%) for 48 h was performed. After stimulation by high glucose (236 mM), cell viability decreased significantly. Glucose concentrations of 83 mM or 138 mM were used in our subsequent experiments (Fig. 1). PTL (1.25–5 μM) did not influence HMrSV5 cell viability, according to our previous report16.
PTL alleviates inflammation in peritoneal fibrosis in vitro and in vivo
Peritoneum inflammation is a key event in the pathogenesis of peritoneal fibrosis23. We evaluated peritoneal inflammation in a non-infected state. The levels of the inflammatory cytokines IL-6, TNF-α, and MCP-1 in long dwell PDF of CAPD patients, omentum tissues of PD mice, and high glucose-treated HMrSV5 cells were measured by ELISA or qRT–PCR. As presented in Fig. 2A–C, the levels of IL-6, TNF-α, and MCP-1 in PDF in the PF group were higher than those in the control group. Similar results were obtained in vitro and in PD mice. The levels of inflammatory cytokines were significantly increased in the high–glucose stimulation group and vehicle group, but PTL administration decreased the levels (Fig. 2D–H).
A–C Significant differences in IL-6, TNF-α, and MCP-1 levels in the long dwell PDF between the PF group (n = 15) and control group (n = 15) as assessed by ELISA. **P < 0.01 versus control group. D, E Real-time-PCR analysis of peritoneal IL-6 and MCP-1 mRNA expression in PD mice (n = 5 per group). *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus PD mice receiving vehicle treatment at day 28. F–H Real-time-PCR analysis of IL-6, TNF-α, and MCP-1 mRNA expression in high glucose- and PTL-treated HMrSV5 cells after 24-h exposure. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05, ***P < 0.001 versus normal controls; #P < 0.05, ###P < 0.001versus glucose-treated cells.
PTL inhibits high glucose-induced MMT in vitro
We then tested the effect of high glucose on HMrSV5 cells and glucose (83 or 138 mM), but not mannitol (138 mM) induced MMT (Fig. 3A, B). Upon high glucose stimulation, different concentrations of PTL (1.25, 2.5, and 5 μM) attenuated high glucose-induced MMT, as shown by decreased levels of fibronectin, collagen I, α-SMA, and increased E-cadherin expression (Fig. 3C–F). To further confirm the effect of PTL in vitro, we isolated RPMCs and verified them by immunofluorescence staining. As presented in Fig. 4A, B, the cells showed a cobblestone-like appearance, were tightly connected, and expressed CK18 and vimentin. The α-SMA staining was barely visible. Similarly, PTL also inhibited high glucose-induced MMT in RPMCs (Fig. 4C–F).
A Western blot analysis of collagen I and α-SMA expression after treatment with glucose (83, 138 mM) or mannitol (138 mM) for 48 h. C Western blot analysis of fibronectin, collagen I, E-cadherin, and α-SMA expression after cotreatment with glucose and PTL for 48 h. B Relative protein expression shown in A. D–G Relative protein expression shown in C. The data are expressed as the mean ± SD of three independent experiments. **P < 0.01, ****P < 0.0001 versus normal controls; ##P < 0.01, ###P < 0.001, ####P < 0.0001 versus glucose (138 mM)-treated cells.
A RPMCs were observed under a light microscope. The scale bar presents 100 μm. B Immunofluorescence staining was used to detect the expression of CK18, vimentin, and α-SMA in RPMCs. The scale bar presents 100 μm. C Western blot analysis of fibronectin, collagen I, and E-cadherin expression after cotreatment with glucose and PTL for 48 h. D–F Relative protein expression shown in C. The data are expressed as the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 versus normal controls; #P < 0.05, ##P < 0.01 versus glucose (138 mM)-treated cells.
PTL decreases the expression of TGF-β1 by inhibiting the NF-κB signaling pathway in vitro and in vivo
The peritoneal MMT induced by TGF-β1 is a key process in progressive peritoneal fibrosis11. We previously reported that PTL could inhibit the phosphorylation of Smad2/3 and affect the level of p65 protein transported to the nucleus16. Therefore, we further detected the expression levels of TGF-β1 and NF-κB signaling pathway molecules in mice and cells through western blot, immunohistochemistry, and immunofluorescence assays.
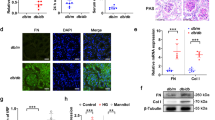
The protein levels of p-IκBα/IκBα, p-p65/ p65, and TGF-β1 were significantly increased in the omentum of PD mice compared with controls, and PTL treatment significantly decreased these levels (Fig. 5A–D). Parietal peritoneum samples of patients who had PD for more than 5 years showed increased expression of TGF-β1 and p65 and thickening compared with those in the normal group (Fig. 5E, F). However, PTL treatment reduced the expression of TGF-β1 and p65 compared to that in mice treated with PD plus vehicle (Fig. 5G).
A Western blot analysis of p-IκBα, IκBα, p-p65, p65 and TGF-β1 expression in PD mice (n = 5). B Relative levels of p-IκBα, p-p65, and TGF-β1 protein shown in A. *P < 0.05, **P < 0.01 versus control group; #P < 0.05, ##P < 0.01versus PD mice receiving vehicle treatment. C Masson’s trichrome staining of peritoneum samples from CAPD patients. The scale bar presents 200 μm. D Immunostaining of p65 and TGF-β1 deposited in the submesothelial area of parietal peritoneum samples from CAPD patients. The scale bar presents 50 μm. E Immunostaining of p65 and TGF-β1 deposited in the submesothelial area of the parietal peritoneum samples from PD mice. The scale bar presents 50 μm.
Similarly, glucose cultures (138 mM) significantly elevated the levels of TGF-β1, p-IκBα/IκBα, and p-p65/ p65 in HMrSV5 cells (Fig. 6A, B, Fig. 7A, C–E), and RPMCs (Fig. 7B, C–E). Cotreatment with different concentrations of PTL (1.25, 2.5, and 5 μM) decreased these levels in a dose-dependent manner. The immunofluorescence staining revealed that glucose (138 mM) made p65 nuclear translocation, which was accompanied by a significant increase in the expression of TGF-β1. In contrast, PTL (5 μM) cotreatment inhibited p65 nuclear translocation and decreased TGF-β1 expression (Fig. 6C).
HMrSV5 cells or RPMCs were coincubated with glucose (83 or 138 mM) plus PTL at different concentrations (0, 1.25, 2.5, 5 μM) for 24 h. A Western blot analysis of p-p65, p65, and TGF-β1 levels in HMrSV5 cells. C Immunofluorescence staining to detect the expression of p65 and TGF-β1 in HMrSV5 cells. The scale bar represents 100 μm. D Western blot analysis of p-IκBα, IκBα, p-p65, p65 and TGF-β1 expression in HMrSV5 cells. E Western blot analysis of p-IκBα, IκBα, p-p65, p65 and TGF-β1 expression in RPMCs. B Relative protein expression shown in A. F–H Relative levels of p-IκBα, p-p65, and TGF-β1 protein shown in D, E. *P < 0.05, **P < 0.01 versus normal controls; #P < 0.05, ##P < 0.01 versus glucose (138 mM)-treated cells.
A The optimal recognition sequence of NF-κBp65 (RELA). B, C ChIP was used to identify NF-κBp65 binding sites within the putative TGF-β1 promoter after treatment with glucose (138 mM) for 24 h. D, E ChIP was used to identify NF-κBp65 binding sites within the putative TGF-β1 promoter after cotreatment with glucose (138 mM) and PTL (5 μM) for 24 h. *P < 0.05, **P < 0.01, ***P < 0.001 versus IgG groups. F Relative enrichment of NF-κBp65 between the treatment of glucose (138 mM) and glucose (138 mM) +PTL (5 μM). G Luciferase reporter assays were used to detect the effect of p65 on TGF-β1 expression. *P < 0.05 compared with PGL3-TGFβ1, #P < 0.05 compared with PGL3- TGFβ1 + pcDNA3.1-p65.
PTL regulates the transcription of TGF-β1 induced by high glucose through NF-κBp65
To determine whether transcriptional regulation contributes to TGF-β1 upregulation in HMrSV5 cells, we analyzed the response elements of transcription factors located within a three-kilobase region upstream of the first exon of the TGF-β1 gene in the UCSC database (https://genome.ucsc.edu/). Conforming to the optimal recognition sequence of NF-κBp65 (RELA) (Fig. 7A), three putative NF-κBp65 binding sites (TGFβ1-1, TGFβ1-2, and TGFβ1-3) within this region were identified using the JASPAR database (http://jaspar.binf.ku.dk). We further confirmed the direct association of p65 with the TGF-β1 promoter through ChIP. Regardless of whether the cells were treated with glucose (138 mM) or glucose (138 mM) + PTL (5 μM), the ChIP results revealed that p65 bound to TGFβ1-1 sites (Fig. 7B–E). Compared to the glucose treatment, cotreatment with PTL inhibited the TGF-β1 promoter targeted by NF-κBp65 (Fig. 7F).
To further investigate the effect of p65 on TGF-β1expression, we performed a luciferase reporter assay. As expected, TGFβ1-1 promoter-driven luciferase activity was much higher, and p65 significantly enhanced luciferase activity driven by the TGFβ1-1 promoter in HMrSV5 cells. However, PTL significantly weakened this luciferase activity (Fig. 7G). These results demonstrated that NF-κBp65 (RELA) can directly bind to the TGF-β1 promoter to regulate the expression of TGF-β1 transcriptionally and that PTL can inhibit this process.
Discussion
We demonstrated that PTL can inhibit the inflammatory state of PD-associated peritoneal fibrosis and decrease the expression of TGF-β1 by inhibiting the NF-κB signaling pathway in vitro and in vivo. This suggests that PTL inhibits NF-κB-targeting of the TGF-β1 promoter, thus inhibiting the effect of the TGF-β/Smad2/3 signaling pathway in PD-related peritoneal fibrosis.
Peritoneal fibrosis is the main cause of peritoneal failure. During the fibrosis process, cells undergo MMT during PD, and the transformed mesothelial cells can produce an extracellular matrix and cause fibrosis8,24. TGF-β1 is a major profibrotic cytokine that plays a key role in progressive peritoneal fibrosis11,25,26. The TGF-β/Smad signaling pathway has always been the focus of research on the mechanism of peritoneal fibrosis27,28. In this pathway, Smad2 and Smad3 are activated by phosphorylation of TβRI. Smad2/3 are released from the receptor complex, form a complex with Smad4, and cooperate with various coactivators to move into the nucleus to regulate the transcription of target genes29. Our previous research showed that suppression of Smad2/3 phosphorylation, by PTL, prevented PD-related peritoneal fibrosis in vitro and in vivo16. In this study, we used glucose (138 mM) at the same concentration as conventional PDF (2.5%) to treat HMrSV5 cells and RPMCs. PTL can inhibit high glucose-induced MMT, as indicated by decreases in the expression of fibrotic markers (fibronectin, collagen I, α-SMA) and increases in the expression of the epithelial marker E-cadherin. Furthermore, PTL decreased the expression of TGF-β1 in PD mice and high glucose-treated HMrSV5 cells/ RPMCs. Our findings confirmed that PTL improves peritoneal fibrosis by suppressing the TGF-β/Smad pathway.
The fibrosis process itself and the inflammation promoted by the non-physiological characteristics of solution both contribute to peritoneal fibrosis7. Inflammation of the peritoneum caused by a non-infected state is eventually accompanied by MMT triggered by inflammation8, which is considered a key event in the pathogenesis of peritoneal fibrosis23. It was shown that peritoneal injury leads to the activation of inflammatory cells, endothelial cells, and mesothelial cells. These cells can recognize bacterial pathogens through Toll-like receptors, leading to the activation of the NF-κB signaling pathway and the subsequent secretion of many inflammatory cytokines30. The NF-κB pathway plays a key role in immune homeostasis and chronic inflammation and has long been proposed as a potential target for disease therapy31. NF-κB is localized in the cytoplasm of non-stimulated cells and must be transported to the nucleus to function. Proteins of the inhibitory κB family (IκB) serve as inhibitors and regulators of NF-κB activity and bind to NF-κB to mask its nuclear localization signal, which prevents nuclear uptake. Upon stimulation of innate immune and cytokine receptors, phosphorylation of IκB results in its proteasomal degradation and the release of NF-κB. NF-κB can then translocate to the nucleus and activate gene transcription32,33. Our data verified the inflammatory state of PD-associated peritoneal fibrosis in CAPD patients, PD mice, and high glucose treated cells. Intervention with PTL alleviated the inflammatory responses of peritoneal fibrosis, which decreased IL-6, TNF-α, and MCP-1levels. Moreover, PTL significantly decreased the phosphorylation levels of IκBα and NF-κBp65 in vitro and in vivo, thereby inhibiting NF-κB activation. These data showed that PTL can improve peritoneal fibrosis by inhibiting the NF-κB signaling pathway and reducing the production of inflammatory cytokines.
The NF-κB and TGF-β signaling pathways are essential in multiple physiological and pathological processes. Previous studies have found that there is crosstalk between the NF-κB and TGF-β signaling pathways, but the effects are inconsistent19. NF-κB can be activated by TGF-β and mediate transcriptional activation of TGF-β target genes in a variety of cell types34,35,36,37, and can be repressed by TGF-β by a negative feedback loop in human intestinal lamina propria mononuclear cells38. In contrast, NF-κBp65 can inhibit TGF-β/Smad signaling by inducing Smad7 expression39. The suppression of the NF-κB/TGF-β/MMP-9 pathway can attenuate inflammation and fibrosis in radiation enteropathy40, but the specific mechanism linking NF-κB and TGF-β is not fully clear. PTL can effectively inhibit inflammation and the NF-κB pathway by directly binding to NF-κB subunit p6512,17. After cotreatment with or without PTL, the changes in the levels of TGF-β1 expression were consistent changes in p-p65/p65 or p65 determined by western blot, immunohistochemistry, and immunofluorescence in vitro and in vivo. Therefore, we speculate that PTL decreases the expression of TGF-β1 by inhibiting the NF-κB signaling pathway. ChIP and luciferase reporter assays were used to confirm the relationship between p65 and TGF-β1. We found that NF-κBp65 can directly bind to the TGF-β1 promoter to transcriptionally regulate the expression of TGF-β1 in HMrSV5 cells treated with high glucose. Compared to the glucose treatment, cotreatment with PTL repressed this binding. These data demonstrated that NF-κBp65 transcriptionally regulates the up-regulation of TGF-β1 in PD-related peritoneal fibrosis, and PTL ameliorates peritoneal fibrosis by inhibiting the NF-κBp65-regulated TGF/Smad signaling pathway.
In summary, PTL significantly alleviates peritoneal fibrosis in PD mice and TGF-β or high glucose-treated HMrSV5 cells/RPMCs. PTL has a therapeutic effect on peritoneal fibrosis by inhibiting inflammation caused by the NF-κB/TGF-β/Smad signaling axis.
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Webster, AC, Nagler, EV, Morton, RL, Masson, P. Chronic kidney disease. Lancet 389,1238-1252(2017)
Romagnani, P, Remuzzi, G, Glassock, R, Levin, A, Jager, KJ, Tonelli, M, et al. Chronic kidney disease. Nat Rev Dis Primers 3,17088(2017)
Zhang, L, Zhao, MH, Zuo, L, Wang, Y, Yu, F, Zhang, H, et al. China Kidney Disease Network (CK-NET) 2015 Annual Data Report. Kidney Int Suppl 9, e1-1e81(2019)
Purnell, TS, Auguste, P, Crews, DC, Lamprea-Montealegre, J, Olufade, T, Greer, R, et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am J Kidney Dis 62, 953-73(2013)
Kaplan, AA. Peritoneal dialysis or hemodialysis: present and future trends in the United States. Contrib Nephrol 189, 61-64(2017)
Li, PK, Chow, KM, Van de Luijtgaarden, MW, Johnson, DW, Jager, KJ, Mehrotra, R, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol 13, 90–103(2017)
Zhou, Q, Bajo, MA, Del Peso, G, Yu, X, Selgas, R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int 90, 515-524(2016)
de Lima, SM, Otoni, A, Sabino Ade, P, Dusse, LM, Gomes, KB, Pinto, SW, et al. Inflammation, neoangiogenesis and fibrosis in peritoneal dialysis. Clin Chim Acta 421,46-50(2013)
Devuyst, O, Margetts, P J, Topley, N. The pathophysiology of the peritoneal membrane. J Am Soc Nephrol 21,1077-1085(2010)
Si, M, Wang, Q, Li, Y, Lin, H, Luo, D, Zhao, W, et al. Inhibition of hyperglycolysis in mesothelial cells prevents peritoneal fibrosis. Sci Transl Med 11, eaav5341(2019)
López-Cabrera, M. Mesenchymal conversion of mesothelial cells is a key event in the pathophysiology of the peritoneum during peritoneal dialysis. Adv Med 2014, 473134(2014)
Freund, RRA, Gobrecht, P, Fischer, D. Advances in chemistry and bioactivity of parthenolide. Nat Prod Rep 37, 541-565(2020)
Ghantous, A, Sinjab, A, Herceg, Z, Darwiche, N. Parthenolide: from plant shoots to cancer roots. Drug Discov Today 18, 894-905(2013)
Wang, M, Li, Q. Parthenolide could become a promising and stable drug with anti-inflammatory effects. Nat Prod Res 29, 1092-1101(2015)
Majdi, M, Liu, Q, Karimzadeh, G, Malboobi, MA, Beekwilder, J, Cankar, K, et al. Biosynthesis and localization of parthenolide in glandular trichomes of feverfew (Tanacetum parthenium L. Schulz Bip). Phytochemistry 72, 1739–1750(2011)
Zhang, Y, Huang, QY, Chen, YH, Peng, X, Wang, YX, Li, ST, et al. Parthenolide, an NF-κB inhibitor, alleviates peritoneal fibrosis by suppressing the TGF-β/Smad pathway. Int Immunopharmacol 78, 106064(2020)
Bork, PM, Schmitz, ML, Kuhnt, M, Escher, C, Heinrich, M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-kappaB. FEBS Lett 402, 85-90(1997)
Siedle, B, García-Piñeres, AJ, Murillo, R, Schulte-Mönting, J, Castro, V, Rüngeler, P, et al. Quantitative structure-activity relationship of sesquiterpene lactones as inhibitors of the transcription factor NF-kappaB. J Med Chem 47, 6042-6054(2004)
Luo, K. Signaling cross-talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol 9, a022137(2017)
Zhou, Q, Yu, X. Isolation and propagation of rat peritoneal mesothelial cells. Methods Mol Biol 1397, 25-34(2016)
Chen, X, Liu, W, Xiao, J, Zhang, Y, Chen, Y, Luo, C, et al. FOXO3a accumulation and activation accelerate oxidative stress-induced podocyte injury. FASEB J 34, 13300-13316(2020)
Peng, F, Li, H, Li, S, Wang, Y, Liu, W, Gong, W, et al. Micheliolide ameliorates renal fibrosis by suppressing the Mtdh/BMP/MAPK pathway. Lab Invest 99, 1092-1106(2019)
Zhang, Z, Jiang, N, Ni, Z. Strategies for preventing peritoneal fibrosis in peritoneal dialysis patients: new insights based on peritoneal inflammation and angiogenesis. Front Med 11, 349-358(2017)
Strippoli, R, Moreno-Vicente, R, Battistelli, C, Cicchini, C, Noce, V, Amicone, L, et al. Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem Cells Int 2016, 3543678(2016)
Margetts, PJ, Churchill, DN. Acquired ultrafiltration dysfunction in peritoneal dialysis patients. J Am Soc Nephrol 13, 2787-94(2002)
Loureiro, J, Aguilera, A, Selgas, R, Sandoval, P, Albar-Vizcaíno, P, Pérez-Lozano, ML, et al. Blocking TGF-beta1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol 22, 1682–1695(2011)
Xu, J, Lamouille, S, Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 19, 156-72(2009)
Lan, HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci 7, 1056-67(2011)
Biernacka, A, Dobaczewski, M, Frangogiannis, NG. TGF-βsignaling in fibrosis. Growth Factors 29, 196–202(2011)
Yung, S, Chan, TM. Intrinsic cells: mesothelial cells-central players in regulating inflammation and resolution. Perit Dial Int 29, S21–S27(2009)
Barnes, PJ, Karin, M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336, 1066-71(1997)
Karin, M, Ben-Neriah, Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18, 621-63 (2000)
Napetschnig, J, Wu, H. Molecular basis of NF-κB signaling. Annu Rev Biophys 42, 443-68(2013)
Baroni, G, Schuinski, A, de Moraes, TP, Meyer, F, Pecoits-Filho, R. Inflammation and the peritoneal membrane: causes and impact on structure and function during peritoneal dialysis. Mediators Inflamm 2012, 912595(2012)
Brandl, M, Seidler, B, Haller, F, Adamski, J, Schmid, RM, Saur, D, et al. IKK(α) controls canonical TGF(β)-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in panc1 cells. J Cell Sci 123, 4231-9(2010)
Yeh, YY, Chiao, CC, Kuo, WY, Hsiao, YC, Chen, YJ, Wei, YY, et al. TGF-beta1 increases motility and alphavbeta3 integrin up-regulation via PI3K, Akt and NF-kappaB-dependent pathway in human chondrosarcoma cells. Biochem Pharmacol 75, 1292-301(2008)
Ishinaga, H, Jono, H, Lim, JH, Kweon, SM, Xu, H, Ha, UH, et al. TGF-beta induces p65 acetylation to enhance bacteria-induced NF-kappaB activation. EMBO J 26, 1150-62(2007)
Monteleone, G, Mann, J, Monteleone, I, Vavassori, P, Bremner, R, Fantini, M, et al. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-κB activation in gut inflammation. J Biol Chem 279, 3925-32(2004)
Freudlsperger, C, Bian, Y, Contag Wise, S, Burnett, J, Coupar, J, Yang, X, et al. TGF-β and NF-κB signal pathway crosstalk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 32, 1549-59(2013)
Mohamed, HA, Said, RS. Coenzyme Q10 attenuates inflammation and fibrosis implicated in radiation enteropathy through suppression of NF-kB/TGF-β/MMP-9 pathways. Int Immunopharmacol 92, 107347(2021)
Acknowledgements
We would like to thank Professor Xueqing Yu for providing the HMrSV5 cells, Accendatech Co., Ltd. (Tianjin, China) for providing PTL, and Central Laboratory in the Second Affiliated Hospital of Guangzhou Medical University for providing experimental sites.
Funding
We acknowledge the financial support provided by the Medical Scientific Research Foundation of Guangdong Province, China (No. A2021398), the Basic and Applied Basic Research Foundation of Guangdong Province, China (No. 2022A1515012665), the National Natural Science Foundation of China (NSFC) (no. 81800612), the Natural Science Foundation of Guangdong Province, China (No. 2020A1515011295), University Scientific Research Project of Guangzhou Education Bureau (No. 201831833).
Author information
Authors and Affiliations
Contributions
JL, HL, and YH performed study concept and design; YZ, WF, XP, and LZ performed development of methodology and writing, review, and revision of the paper; ZW, HS, CC, LX, YZ, and ML provided acquisition, analysis and interpretation of data, and statistical analysis; YZ, XP, and SL provided technical and material support. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experimental procedures were approved by the second Affiliated Hospital of Guangzhou Medical University. The studies were performed in accordance with the Declaration of Helsinki and to the Guide for the Care and Use of Laboratory Animals designated by the National Research Council.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Feng, W., Peng, X. et al. Parthenolide alleviates peritoneal fibrosis by inhibiting inflammation via the NF-κB/ TGF-β/Smad signaling axis. Lab Invest 102, 1346–1354 (2022). https://doi.org/10.1038/s41374-022-00834-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-022-00834-3
This article is cited by
-
Brahma-related gene 1 acts as a profibrotic mediator and targeting it by micheliolide ameliorates peritoneal fibrosis
Journal of Translational Medicine (2023)
-
Recent advances in anti-inflammatory active components and action mechanisms of natural medicines
Inflammopharmacology (2023)