Abstract
Exosomes, one of three main types of extracellular vesicles, are ~30–100 nm in diameter and have a lipid bilayer membrane. They are widely distributed in almost all body fluids. Exosomes have the potential to regulate unknown cellular and molecular mechanisms in intercellular communication, organ homeostasis, and diseases. They are critical signal carriers that transfer nucleic acids, proteins, lipids, and other substances into recipient cells, participating in cellular signal transduction and material exchange. ncRNAs are non-protein-coding genes that account for over 90% of the genome and include microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs). ncRNAs are crucial for physiological and pathological activities in the liver by participating in gene transcription, posttranscriptional epigenetic regulation, and cellular processes through interacting with DNA, RNA, or proteins. Recent evidence from both clinical and preclinical studies indicates that exosome-derived noncoding RNAs (ncRNAs) are highly involved in the progression of acute and chronic liver diseases by regulating hepatic lipid metabolism, innate immunity, viral infection, fibrosis, and cancer. Therefore, exosome-derived ncRNAs have promising potential and clinical implications for the early diagnosis, targeted therapy, and prognosis of liver diseases.
Similar content being viewed by others
Introduction
The pathogenesis of liver diseases is very complex and involves a variety of pathogenic factors, including hepatitis virus infection, immune dysregulation, drug abuse, excessive alcohol consumption, and obesity. The high prevalence of nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), and viral hepatitis has become the most common cause of chronic liver disease1. Furthermore, more than 257 million people worldwide have chronic hepatitis B (CHB) virus infection2. If the pathogenic factors persist long-term, liver injury progresses to fibrosis, cirrhosis, and even cancer. Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, accounting for ~75–85% of primary liver cancer cases3.
Exosomes can be secreted from parenchymal cells (hepatocytes) and nonparenchymal cells (including hepatic stellate cells (HSCs), bile duct cells, Kupffer cells, and hepatic endothelial cells) under physiological and pathological conditions4. Several studies have highlighted the key roles of exosomes in cell communication, as exosomes can transfer functional substances, especially noncoding RNAs (ncRNAs), into recipient cells to regulate cell biological properties5.
In this review, we summarize the clinical significance and potential of exosomal ncRNAs, which provides us with a better understanding of the initiation of, development of, and interventions for liver diseases.
Biological characteristics of exosomes
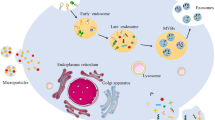
Exosomes are small vesicles with a phospholipid bilayer membrane and a diameter of 30–100 nm6; exosomes can be released by B lymphocytes, T cells, mast cells, dendritic cells, tumor cells, endothelial cells, mesenchymal stem cells, and other cells7. They are widely distributed in serum, urine, bile, saliva, semen, cerebrospinal fluid, and breast milk8. Exosomes are mainly derived from intracellular multivesicular bodies (MVBs) by the “endocytosis-fusion-efflux” process. The process is as follows9: (1) The cell membrane invaginates to form endosomes, which fuse to form early endosomes; (2) the microparticle membranes of early endosomes are invaginated to form late endosomes, also called MVBs, enclosing intracellular fluids or biomolecules; and (3) these multiple intracavitary vesicles are degraded by fusion with lysosomes or cell membranes and are finally released into the extracellular matrix (ECM) (Fig. 1).
Exosomes contain a large number of biomacromolecules, including proteins (heat shock proteins and tetraspanins), nucleic acids (mRNA, ncRNA, and DNA), and lipids (cholesterol)10. Exosomes can directly interact with signaling receptors on target cells, and plasma membrane fusion then promotes the delivery of bioactive components into the cytoplasm5,10, a process called receptor-mediated endocytosis and phagocytosis.
Biological characteristics of ncRNAs
Protein-coding sequences account for <2% of the human genome; however, more than 90% of other sequences are located in noncoding regions11. These non-protein-coding genes are replicated to produce thousands of RNA molecules named ncRNAs, including microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs). NcRNAs, which are crucial for physiological and pathological activities in the liver, can regulate gene transcription, posttranscriptional epigenetic modifications, and cellular processes by interacting with DNA, RNA, or proteins12.
The length of miRNAs is ~20–24 nucleotides, and miRNAs mainly regulate protein translation by inhibiting posttranscriptional processes13. MiRNAs can target a complementary sequence in the 3′UTRs of mRNAs by base pairing (bp). MiR-122 is the most abundant microRNA in the liver, accounting for ~70% of the total miRNA population14. Some studies have shown that miR-122 strongly participates in liver homeostasis15.
LncRNAs are ncRNAs with a transcript length of more than 200 bp. LncRNAs were initially, albeit wrongly, considered a byproduct of RNA polymerase II transcription, i.e., the “noise and garbage” of gene transcription with no biological function. However, recent studies have shown that lncRNAs can affect gene expression in the development of liver disease at various levels through DNA methylation, histone modification, posttranscriptional regulation, and RNA interference16,17.
CircRNAs originate from precursor mRNA transcripts and are produced via a special alternative splicing mechanism called “backsplicing”18. Unlike lncRNAs, circRNAs are covalently closed loops. Due to their lack of typical terminal structures (3′- and 5′-terminal regions), they are more stable than lncRNAs. CircRNAs can also act as molecular sponges of miRNAs, negatively regulating the expression of miRNAs and participating in splicing, transcriptional, and posttranscriptional events, as well as host gene expression, in the initiation and progression of liver disease18.
Nonalcoholic fatty liver disease (NAFLD)
With the prevalence of obesity and other components of metabolic syndrome, NAFLD has become a common chronic liver disease. Recent studies have shown that exosomes play an important role in regulating injury, amplifying inflammation, and promoting fibrosis in NAFLD19. Exosomes from hepatocytes were enriched with an abundance of miR-122 and miR-192 after mice were fed a choline-deficient L-amino acid diet19. After miR-155 knockout mice were fed a methionine–choline-deficient or a methionine–choline-sufficient diet for 5 weeks, miR-155 deficiency was found to alleviate hepatic steatosis20. These studies indicate that miRNAs can be used as potential candidate markers and therapeutic targets for NAFLD.
Alcoholic fatty liver disease (AFLD)
Alcohol addiction is a social health issue that directly leads to AFLD, which progresses to fibrosis, cirrhosis, and even cancer. AFLD progression has been suggested to be associated with exosome-derived ncRNAs. The miR-155 level was found to be increased in ALD patients and chronic alcohol-fed mice and can promote exosome release from both macrophages and hepatocytes by downregulating autophagic proteins, including mTOR, Rheb, LAMP1, and LAMP221. Compared with that in healthy volunteers, the miR-155 level in circulating exosomes was found to be elevated in patients with ALD21. In addition, the expression of miR-let7f, miR-29a, and miR-340 in exosomes released from hepatocytes was increased in a mouse model of mild alcoholic steatohepatitis induced by treatment with ethanol for four weeks but not in other mouse models, including bile duct ligation, nonalcoholic steatohepatitis, and obese mouse models. These results indicate that the above miRNAs can be used as a “barcode” to distinguish ALD due to their specificity22.
Furthermore, in a mouse model of alcohol-induced steatohepatitis, the number of circulating exosomes was increased significantly after intragastric administration of alcohol23. Through microarray screening of exosomes, the miR-192, miR-122, and miR-30a levels in the serum of chronic alcohol-fed mice were found to be increased. Moreover, increased levels of miR-192 and miR-30a were observed in humans with alcoholism and hepatitis, indicating their diagnostic value for alcoholic hepatitis23. A previous study showed that an increase in circulating miR-122 is associated with liver injury regardless of the cause of liver injury24. Serum exosome-derived miR-122 from hepatocytes can be absorbed by macrophages after alcohol drinking or ethanol ingestion; this event can reprogram monocytes and induce sensitization to lipopolysaccharide, thus promoting the inflammatory response in ALD25.
Moreover, alcohol-induced monocytes can secrete exosomes to deliver miR-27a into non-alcohol-induced monocytes, which can subsequently differentiate into M2 macrophages with expression of inflammatory IL-10 and TGF-β126. Exosomes from alcohol-treated hepatocytes expressing the CD40 ligand can promote macrophage M2 activation in a caspase-dependent manner, which can also lead to ALD-associated inflammation27. These studies indicate that exosome-derived ncRNAs play an important role in the process of alcohol-induced inflammatory responses and liver injury.
Viral hepatitis
Exosomes are highly involved in the pathogenesis of viral hepatitis through virus transmission, immune regulation, antiviral response control, and microenvironmental manipulation (including intracellular material secretion as well as intercellular exchange of material and information)28,29,30.
Hepatitis C virus (HCV)
By isolating exosomes from individuals infected with hepatitis C virus (HCV), these exosomes were found to contain replication-competent viral RNA in a complex with Ago2-miR122-HSP90. More importantly, exosomes loaded with an miR-122 inhibitor can inhibit HCV transmission mediated by exosomes from hepatocytes28. This indicates that targeting exosome-derived miRNAs can inhibit HCV transmission.
Hepatitis B virus (HBV)
Hepatitis B virus (HBV)-infected hepatocytes can activate NK cells by releasing exosomal miR-21 and downregulating IL-12 expression, which might be critical for viral escape from the host innate immune response29. The expression of exosome-derived HBV-miR-3 is positively correlated with the serum HBV titer in patients with HBV infection at the acute stage. HBV-miR-3 inhibits the expression of HBsAg and HBeAg as well as HBV replication by targeting a 3.5 kb region of the core protein mRNA30. The levels of exosome-derived miR-192-5p, miR-193b-3p, miR-194-5p, miR-122, and miR-22 are significantly higher in HBeAg-positive patients and are correlated with the HBV DNA level and HBsAg titer31,32. Therefore, exosome-derived ncRNAs can also be used as candidate biomarkers for HBV infection.
Autoimmune liver diseases (AILDs)
Autoimmune liver diseases (AILDs) are a group of chronic inflammatory hepatic disorders that includes autoimmune hepatitis (AIH), primary biliary cholangitis, and primary sclerosing cholangitis33. Recent investigations of ncRNA expression profiles indicate that ncRNAs have important impacts on some autoimmune diseases. MiR-223 is highly expressed in bone marrow-derived mesenchymal stem cells (BMSCs). The expression of cytokines, NLRP3 and caspase-1 is downregulated by BMSC-derived exosomal miR-223 (+) in a mouse model of AIH induced by the liver antigen S100, and this downregulation might protect against liver injury from immune-inflammatory dysregulation34. Interestingly, treatment with exosomes containing miR-223-3p successfully attenuates inflammatory cytokine release in both the liver and macrophages by regulating STAT3 expression35.
Drug-induced liver injury (DILI)
Drug-induced liver injury (DILI) has gradually become a global public health problem and is characterized by elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Exosome-derived ncRNAs might be better diagnostic biomarkers than the current biomarkers. A previous study suggested that acetaminophen (APAP) administration results in a robust increase in miR-122 expression in hepatocytes and a modest increase in the expression of inflammatory miRNAs such as miR-155, miR-146a, and miR-125b in plasma. These miRNA signatures could potentially represent biomarkers for APAP-induced liver injury24. The elevated levels of liver-specific miRNAs such as miR-122, miR-192, and miR-155 in exosomes in APAP-induced liver injury are attenuated by treatment with the antioxidant N-acetylcysteine36. Serum exosome miR-370-3p might be the key factor enhancing the cytotoxic effect of fructus meliae toosendan by elevating p21 and Cyclin E expression37. In summary, profiling circulating exosome-derived ncRNAs could contribute to deepening the understanding of DILI.
Biliary atresia (BA)
Biliary atresia is a progressive biliary disease of unknown etiology. It often occurs in newborns, resulting in intrahepatic and extrahepatic bile duct occlusion and cholestasis38. Exosome-derived ncRNAs have been reported to participate in the development of biliary atresia. For example, the level of serum exosomal lncRNA H19 is correlated with liver fibrosis severity in biliary atresia patients, especially in those with severe liver fibrosis (sixfold higher than in patients with mild liver fibrosis). LncRNA H19 plays a vital role in cholangiocyte proliferation and cholestatic liver injury in biliary atresia by regulating the S1PR2/SphK2 and let-7/HMGA2 axes39.
Cholangiocarcinoma (CCA)
Cholangiocarcinoma is a highly heterogeneous malignant epithelial tumor whose early diagnosis is very difficult40. To date, an increasing number of exosomal miRNAs have been used in the diagnosis of cholangiocarcinoma. For instance, a novel miRNA-based panel (including miR-16, miR-486-3p, miR-484, miR-1274b, and miR-191) from biliary exosomes exhibited a sensitivity of 67% and specificity of 96% for CCA diagnosis. A diagnostic panel based on bile duct carcinoma-associated miRNAs showed potential clinical utility41. Furthermore, 38 miRNAs in CCA cell-derived exosomes are significantly upregulated. The miR-205-5p and miR-200 family members in CCA cell-derived exosomes are markedly upregulated (600–1500-fold). However, CCA cell invasion and migration are reduced by silencing miR-205-5p expression. The observed upregulation of miR-205-5p in CCA cells supports the oncogenic role of miR-205-5p in cholangiocarcinoma42.
Liver fibrosis
Liver fibrosis is caused by excessive production and accumulation of insoluble collagen and ECM components after induction of chronic liver injury by multiple pathological factors. Activation of (HSCs) is the central event in hepatic fibrosis development. The number of exosomes released from healthy hepatocytes is limited, while stress can promote the release of exosomes enriched with ncRNAs by hepatocytes43. These substances regulate transcriptional processes in adjacent hepatocytes and nonparenchymal cells in response to chronic hepatic inflammation, acute injury, and fibrosis44.
For example, miR-214 exported from HSCs via exosomes can be delivered to adjacent cells and then downregulate connective tissue growth factor (CCN2) expression45. CCN2 expression is increased in activated HSCs and directly regulates the activation of HSCs, including the processes of mitosis, chemotaxis, and fibrosis46. It was further found that an E-box in the miR-214 promoter binds to the basic helix–loop–helix transcription factor Twist1, which drives the expression of miR-214 and leads to CCN2 inhibition47. Moreover, exosome-mediated toll-like receptor 3 (TLR3) activation in HSCs aggravates liver fibrosis by enhancing IL-17A expression in TLR3-positive γδ T cells. During liver injury, hepatocytes secrete exosomes that contain diverse types of self ncRNAs, which have been recognized as activators of TLR348.
Exosomes from HCV-infected hepatocytes transfer miR-192 into HSCs, which leads to the activation of HSCs and their transdifferentiation into myofibroblasts via upregulation of TGF-b149. In addition, HCV-infected hepatocytes release exosomal miR-19a to activate the STAT3-mediated TGF-β signaling pathway by targeting SOCS3 in HSCs, which promotes HSC activation and liver fibrosis50. miR-155-knockout mice exhibit reduced expression of collagen and α smooth muscle actin (αSMA)51. In summary, exosomal miRNAs can activate HSCs through a variety of signaling pathways in liver fibrosis.
Hepatocellular carcinoma (HCC)
Liver cancer is the sixth most common malignant tumor and the fourth leading cause of cancer-related death worldwide3. Serum alpha-fetoprotein (AFP) is used for early diagnosis of liver cancer, but its specificity is very low52. Recently, an increasing number of studies have focused on ncRNAs in exosomes as candidate biomarkers for liver cancer, as shown in Fig. 2. NcRNAs, which perform biological functions in cancer cell processes such as tumorigenesis, tumor metastasis, angiogenesis, immune regulation, and drug resistance, can be selectively enriched in exosomes53.
Exosomal ncRNAs which relate to cancer-associated fibroblasts are miR-335-5p, miR-320a, and miR-21. Exosomal ncRNAs could escape from immune surveillance (miR-23a-3p, miR-155), mediate chemotherapy resistance (linc-VLDLR), promote tumor metastasis (circ-DB, circ-PTGR1), promote tumor angiogenesis (miR-155, lncR-H19), regulate macrophage polarization (lncR-TUC339).
MiRNAs and diagnosis and prognosis
MiRNAs are encapsulated in the lipid bilayer membrane of exosomes and are not affected by RNases. MiRNAs exist widely in various body fluids. They can not only be used as markers for early diagnosis of liver cancer but also as promising biomarkers for liver cancer prognosis. Compared with those in healthy volunteers and patients with chronic liver disease, the serum miR-30e and miR-223 levels are decreased in HCC patients, which includes HCV-infected patients with HCC, HBV-infected patients with HCC and patients with non-viral infection-associated HCC54. In addition, an increasing number of studies have indicated that exosome-derived miRNAs can be excellent biomarkers for clinical diagnosis and prognosis. For instance, the levels of miR-18a, miR-221, miR-222, and miR-224 in plasma exosomes are significantly higher in patients with HCC than in patients with CHB or liver cirrhosis. However, plasma exosomal miR-101, miR-106b, miR-122, and miR-195 levels in patients with HCC are significantly lower than those in patients with CHB43. In addition, a high serum miR-103 level is associated with a higher metastatic potential of HCC; a higher level of miR-103 in exosomes is also positively associated with HCC metastasis55. The expression of miR-10b, miR-21, miR-122, and miR-200a is a more significant indicator than the expression of AFP in the early stage of HCC. Different combinations of AFP and exosomal miRNAs can predict HCC better than AFP can56. In one study, the level of miR-665 in patients with HCC was significantly higher than that in healthy subjects, and the survival time in the high expression group was significantly shorter than that in the low expression group, suggesting that serum miR-665 may be a new minimally invasive biomarker for the diagnosis and prognosis of HCC57. The level of exosomal miR-125b in HCC patients is lower than that in CHB and LC patients. The level of miR-125b in exosomes is related to the number of tumors and TNM stage. Patients with a low level of miR-125b have shorter recurrence-free survival and overall survival times. MiR-125b can be used as a promising prognostic marker for HCC58. Circulating exosomal miR-21 is associated with more advanced TNM stage, portal vein thrombosis, and other unfavorable prognostic factors. The overall survival and progression-free survival times are significantly shorter in patients with higher circulating exosomal miR-21 levels59.
MiRNAs and cancer associated fibroblasts (CAFs)
In cancer, HSCs can be hijacked to transform into cancer-associated fibroblasts (CAFs). CAFs promote cancer metastasis. HSC-derived exosomes can transport miR-335-5p to recipient liver cancer cells in vitro and in vivo, inhibiting the proliferation and invasion of liver cancer cells and promoting HCC tumor shrinkage in vivo60. Sequencing of exosomes released from fibroblasts from HCC patients and the corresponding paracancerous fibroblasts showed that the miR-320a level in CAF-derived exosomes was significantly decreased. MiR-320a can inhibit the proliferation, migration, and metastasis of HCC cells by binding to its downstream target PBX361. The miR-320a-PBX3 axis can inhibit tumor progression by blocking MAPK pathway activation61. HCC cell-secreted exosomal miR-21 activates PDK1/Akt signaling to induce the transformation of HSCs into CAFs by directly targeting PTEN, which promotes cancer progression via the secretion of angiogenic cytokines, including VEGF, MMP2, MMP9, bFGF, and TGF-β62.
MiRNAs and angiogenesis
The expression of miR-155 in the exosomes of 40 patients with HCC. MiR-155 is significantly upregulated in both cells and exosomes under hypoxic conditions. miR-155 knockout in HCC cells attenuates the effect of exosomes on angiogenesis in human umbilical vein endothelial cells (HUVECs) under hypoxia, suggesting that exosomal miR-155 may affect angiogenic activity in HCC63.
MiRNAs and immune escape
Alcohol increases the release of exosomes containing miR-155 from hepatocytes and Kupffer cells by reducing LAMP1 and LAMP2 expression in the ALD liver21. Again, upregulating miR-155 can increase exosome release from Kupffer cells and hepatocytes21. Tumor-associated macrophages further promote dysfunction of tumor-infiltrating lymphocytes by expressing the ligands of the inhibitory receptors programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4, leading to immune evasion of cancer cells64. Under endoplasmic reticulum stress conditions, the expression of PTEN is inhibited in HCC cells via transfer of miR-23a-3p to macrophages, thus increasing the protein levels of phosphorylated Akt and PD-L1 in macrophages and inhibiting the function of T cells, finally leading to tumor cell escape immune surveillance65.
LncRNAs and diagnosis and prognosis
Circulating exosomal lncRNA ATB is associated with more advanced TNM stage, portal vein thrombosis, and other unfavorable prognostic factors. The overall survival and progression-free survival times are significantly shorter in patients with higher circulating exosome lncRNA ATB levels59. The lncRNA FAL1 is upregulated in serum exosomes of patients with HCC; FAL1 can be transferred into HCC cells and accelerate HCC cell proliferation and metastasis by competitively binding miR-123666. In patients with HCV-related HCC, the expression of lncRNA-HEIH in serum and exosomes is increased, but the expression of lncRNA-HEIH in serum is lower than that in exosomes, indicating that exosome-derived lncRNA-HEIH is more important than lncRNA-HEIH in serum67. Again, higher levels of serum exosomal lincENSG00000258332.1 and Linc00635 in HCC are associated with portal vein tumor embolization, lymph node metastasis, more advanced TNM stage, and lower overall survival. More importantly, the AUC for lincENSG00000258332.1 and Linc00635 binding to serum AFP is 0.894, indicating that these lincRNAs are good biomarkers for HCC68.
LncRNAs and angiogenesis
Moreover, exosomes from CD90 + liver cells and hepatoma cell lines can regulate endothelial cells and promote angiogenesis and intercellular adhesion, unlike those released from parental hepatoma cells. LncRNA H19 is enriched in exosomes released from CD90 + cells and can affect the tumor microenvironment by promoting angiogenesis69.
LncRNAs and macrophage polarization
LncRNA-TUC339 derived from hepatoma cells plays a key role in macrophages by promoting M2 polarization and reduces the production of proinflammatory cytokines, the expression of costimulatory molecules, and the phagocytic capacity, finally promoting the progression of HCC70.
LncRNAs and chemotherapeutic resistance
Exosomal ncRNAs have been found to be associated with chemotherapeutic resistance. For example, when hepatoma cells are exposed to anticancer agents such as sorafenib, camptothecin, and doxorubicin, the release of exosomal lincRNA-VLDLR (Linc-VLDLR) from HCC cells is obviously increased. Linc-VLDLR increases the expression of ATP binding box and subfamily G member 2 (ABC-g2) and then reduces chemotherapeutic agent-induced apoptosis71.
CircRNAs and HCC metastasis
The exosomal content of the circRNA circ-deubiquitination (exo-circ-DB) is increased in HCC patients with high body fat percentages. In addition, in vitro and in vivo experiments have shown that exo-circ-DB promotes the growth of HCC cells by inhibiting miR-34a and activating deubiquitination-related USP7, effects that can be reversed by circ-DB knockout72. Sequencing of the circRNAs in exosomes from nonmetastatic (HepG2), low-metastatic (97 L), and high-metastatic (LM3) hepatoma cells showed that circPTGR1 is specifically expressed in exosomes from 97 L and LM3 cells. Exosomes from LM3 hepatoma cells can enhance the migration and invasion potential of HepG2 and 97 L cells by carrying circPTGR1. The prognosis of HCC patients with low exosomal circPTGR1 levels is better than that of HCC patients with high expression of circPTGR173. In summary, circRNAs can absorb miRNAs to regulate the progression of liver cancer.
Liver regeneration
Liver regeneration refers to the process of proliferation, migration, and differentiation of various hepatocytes to restore normal liver volume and function through a variety of biologically effective cellular signaling pathways. ncRNAs have been reported to be regulatory players in various cellular processes of liver regeneration. MiRNAs regulate the expression of proproliferative and antiproliferative genes to precisely coordinate the proliferation of hepatocytes74. For example, human umbilical cord blood mesenchymal stem cell (hUCB-MSC)-derived exosomal miR-124 enhances liver regeneration by inhibiting Foxg1 in rats after partial hepatectomy75. MiR-10a can accelerate liver regeneration through downregulation of erythropoietin-producing hepatocellular receptor A4 (EphA4)76. These findings can be utilized to develop novel therapies for liver regeneration.
Intervention and perspectives
Understanding how exosomes promote the initiation and progression of liver diseases can help to develop exosome-based therapeutics for hepatic treatment. Although ncRNAs lack the potential to encode proteins, they can affect the expression and functions of other genes through a variety of mechanisms, as shown in Table 1. In some cases, their mechanisms of action are well known, and strategies for controlling their activity by knockin or knockout are well established. Currently, miravirsen (SPC3649), which can bind to the stem–loop structure of pri- and pre-miR-122 with nanomolar affinity and inhibit both Dicer- and Drosha-mediated processing of miR-122 precursors, is in a stage II clinical trial for the treatment of HCV infections77. Targeting ncRNAs may become a novel strategy for the treatment of hepatic diseases.
Exosomes have great potential as delivery vehicles due to their natural substance transport ability, inherent long-term circulation ability, and excellent biocompatibility. They are suitable for delivering ncRNAs to improve their targeting accuracy and provide opportunities for diagnosis and treatment innovation. Qu et al. found that exosomes secreted by adipose-derived mesenchymal stem cells (ADSCs) can be used to transport miRNAs into HSCs. Exosomes from ADSCs have been engineered to overexpress miR-181-5p, which activates autophagy by downregulating STAT3 and Bcl-2 in hst-t6 cells. Subsequently, upregulation of TGF-β1-induced fibrotic gene expression in HST-T6 cells is inhibited, which significantly downregulates type I collagen, vimentin, α-SMA, and fibronectin in the liver and reduces the occurrence of liver fibrosis78. MiR-122-modified exosomes from AMSCs (amsc-122) can enhance the therapeutic effect of AMSCs on CCl4-induced liver fibrosis by inhibiting the activation of HSCs and reducing collagen deposition. Amsc-122 can effectively inhibit proliferation and collagen maturation in HSCs79. Exosomes carrying miR-122 can improve sorafenib resistance in HCC80. Moreover, exosomes carrying miR-17 significantly ameliorate acute liver failure in mice81. Therefore, exosomes loaded with miRNAs might be a new potential strategy for the treatment of liver diseases.
Discussion
Exosome-derived ncRNAs have been shown to have some potential for early diagnosis and prognosis (Table 2). For example, plasma exosome-derived miR-122 has diagnostic sensitivity in multiple hepatic diseases and might be valuable in replacing AFP detection. Some specific ncRNAs need to be further clarified for clinical application by evaluation in larger clinical sample sizes, for example, miR-let7f, miR-29a, and miR-340 for mild AFLD22. Studies on circRNAs should be given more attention, as exosome-derived circRNAs are more stable for diagnosis than miRNAs and lncRNAs due to their lack of a typical terminal structure. Again, as molecular sponges for miRNAs, circRNAs can target miRNAs involved in the progression of liver diseases. More importantly, targeting exosome-derived ncRNAs and exosomes as effective carriers loaded with ncRNAs or siRNAs for precision medicine is an extremely valuable and revolutionary approach for the future.
Data availability
Some or all data used during the study are available from the corresponding author by request.
References
Lindenmeyer, C. C. & McCullough, A. J. The Natural History of Nonalcoholic Fatty Liver Disease-An Evolving View. Clin. Liver Dis. 22, 11–21 (2018).
Lee, H. M. & Banini, B. A. Updates on Chronic HBV: current challenges and future goals. Curr. Treat. Options Gastroenterol. 17, 271–291 (2019).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Sung, S., Kim, J. & Jung, Y. Liver-Derived Exosomes and Their Implications in Liver Pathobiology. Int. J. Mol. Sci. 19, 3715 (2018).
Zhang, J. et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genom. Proteom. Bioinformatics 13, 17–24 (2015).
Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 126, 1208–1215 (2016).
Kogure, T., Lin, W. L., Yan, I. K., Braconi, C. & Patel, T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology 54, 1237–1248 (2011).
Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Urbanelli, L. et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes (Basel) 4, 152–170 (2013).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996).
Rolle, K. et al. The Sequence and Structure Determine the Function of Mature Human miRNAs. PLoS ONE 11, e0151246 (2016).
Holoch, D. & Moazed, D. RNA-mediated epigenetic regulation of gene expression. Nat. Rev. Genet. 16, 71–84 (2015).
Ruan, K., Fang, X. & Ouyang, G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett 285, 116–126 (2009).
Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 (2002).
Higashitsuji, H. et al. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat. Med. 6, 96–99 (2000).
Clark, M. B. et al. Genome-wide analysis of long noncoding RNA stability. Genome. Res. 22, 885–898 (2012).
Ponting, C. P., Oliver, P. L. & Reik, W. Evolution and functions of long noncoding RNAs. Cell 136, 629–641 (2009).
Fanale, D., Taverna, S., Russo, A. & Bazan, V. Circular RNA in Exosomes. Adv. Exp. Med. Biol. 1087, 109–117 (2018).
Povero, D. et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS ONE 9, e113651 (2014).
Csak, T. et al. MicroRNA-155 Deficiency Attenuates Liver Steatosis and Fibrosis without Reducing Inflammation in a Mouse Model of Steatohepatitis. PLoS ONE 10, e0129251 (2015).
Babuta, M. et al. Dysregulated Autophagy and Lysosome Function Are Linked to Exosome Production by Micro-RNA 155 in Alcoholic Liver Disease. Hepatology 70, 2123–2141 (2019).
Eguchi, A. et al. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology 65, 475–490 (2017).
Momen-Heravi, F. et al. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Transl. Med. 13, 261 (2015).
Bala, S. et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 56, 1946–1957 (2012).
Momen-Heravi, F., Bala, S., Kodys, K. & Szabo, G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci. Rep. 5, 9991 (2015).
Saha, B., Momen-Heravi, F., Kodys, K. & Szabo, G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. J. Biol. Chem. 291, 149–159 (2016).
Verma, V. K. et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J. Hepatol. 64, 651–660 (2016).
Bukong, T. N., Momen-Heravi, F., Kodys, K., Bala, S. & Szabo, G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 10, e1004424 (2014).
Kouwaki, T. et al. Extracellular Vesicles Including Exosomes Regulate Innate Immune Responses to Hepatitis B Virus Infection. Front. Immunol. 7, 335 (2016).
Yang, X. et al. Hepatitis B Virus-Encoded MicroRNA Controls Viral Replication. J. Virol. 91, e01919–16 (2017).
Arataki, K. et al. Circulating microRNA-22 correlates with microRNA-122 and represents viral replication and liver injury in patients with chronic hepatitis B. J. Med. Virol. 85, 789–798 (2013).
van der Ree, M. H. et al. Plasma MicroRNA Levels Are Associated With Hepatitis B e Antigen Status and Treatment Response in Chronic Hepatitis B Patients. J. Infect. Dis. 215, 1421–1429 (2017).
Mack, C. L. et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the study of liver diseases. Hepatology 72, 671–722 (2020).
Chen, L. et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol. Immunol. 93, 38–46 (2018).
Lu, F. B. et al. Attenuation of Experimental Autoimmune Hepatitis in Mice with Bone Mesenchymal Stem Cell-Derived Exosomes Carrying MicroRNA-223-3p. Mol. Cells 42, 906–918 (2019).
Cho, Y. E., Kim, S. H., Lee, B. H. & Baek, M. C. Circulating plasma and exosomal microRNAs as indicators of drug-induced organ injury in rodent models. Biomol. Ther. (Seoul) 25, 367–373 (2017).
Zheng, J., Yu, L. Q., Chen, W., Lu, X. Y. & Fan, X. H. Circulating exosomal microRNAsreveal the mechanism of Fructus Meliae Toosendan-induced liver injury in mice. Sci. Rep. 8, 2832 (2018).
Hartley, J. L., Davenport, M. & Kelly, D. A. Biliary atresia. Lancet 374, 1704–1713 (2009).
Xiao, Y. et al. Long Noncoding RNA H19 Contributes to Cholangiocyte Proliferation and Cholestatic Liver Fibrosis in Biliary Atresia. Hepatology 70, 1658–1673 (2019).
Forner, A. et al. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver. Int. 39, 98–107 (2019).
Li, L. et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology 60, 896–907 (2014).
Kitdumrongthum, S. et al. Dysregulated microRNA expression profiles in cholangiocarcinoma cell-derived exosomes. Life Sci. 210, 65–75 (2018).
Sohn, W. et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp. Mol. Med. 47, e184 (2015).
Chen, L., Chen, R., Kemper, S. & Brigstock, D. R. Pathways of production and delivery of hepatocyte exosomes. J Cell Commun. Signal 12, 343–357 (2018).
Chen, L. et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 59, 1118–1129 (2014).
Huang, G. & Brigstock, D. R. Regulation of hepatic stellate cells by connective tissue growth factor. Front. Biosci. (Landmark Ed) 17, 2495–2507 (2012).
Chen, L., Chen, R., Kemper, S., Charrier, A. & Brigstock, D. R. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. Am. J. Physiol. Gastrointest. Liver Physiol. 309, 491–499 (2015).
Seo, W. et al. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology 64, 616–631 (2016).
Kim, J. H., Lee, C. H. & Lee, S. W. Exosomal Transmission of MicroRNA from HCV Replicating Cells Stimulates Transdifferentiation in Hepatic Stellate Cells. Mol. Ther. Nucleic Acids 14, 483–497 (2019).
Devhare, P. B. et al. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J. Virol. 91, e02225–16 (2017).
Bala, S. et al. Biodistribution and function of extracellular miRNA-155 in mice. Sci. Rep. 5, 10721 (2015).
Jeon, Y., Jang, E. S., Choi, Y. S., Kim, J. W. & Jeong, S. H. Glypican-3 level assessed by the enzyme-linked immunosorbent assay is inferior to alpha-fetoprotein level for hepatocellular carcinoma diagnosis. Clin. Mol. Hepatol. 22, 359–365 (2016).
Fan, Q. et al. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett. 414, 107–115 (2018).
Bhattacharya, S. et al. Serum miR-30e and miR-223 as Novel Noninvasive Biomarkers for Hepatocellular Carcinoma. Am. J. Pathol. 186, 242–247 (2016).
Fang, J. H. et al. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 68, 1459–1475 (2018).
Liu, W. H. et al. Combination of exosomes and circulating microRNAs may serve as a promising tumor marker complementary to alpha-fetoprotein for early-stage hepatocellular carcinoma diagnosis in rats. J. Cancer Res. Clin. Oncol. 141, 1767–1778 (2015).
Qu, Z. et al. Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget 8, 80666–80678 (2017).
Liu, W. et al. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. Onco. Targets Ther. 10, 3843–3851 (2017).
Lee, Y. R. et al. Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int. J. Cancer 144, 1444–1452 (2019).
Wang, F., Li, L., Piontek, K., Sakaguchi, M. & Selaru, F. M. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology 67, 940–954 (2018).
Zhang, Z. et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 397, 33–42 (2017).
Zhou, Y. et al. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J. Exp. Clin. Cancer Res. 37, 324 (2018).
Matsuura, Y. et al. Exosomal miR-155 Derived from Hepatocellular Carcinoma Cells Under Hypoxia Promotes Angiogenesis in Endothelial Cells. Dig. Dis. Sci. 64, 792–802 (2019).
Petty, A. J. & Yang, Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy 9, 289–302 (2017).
Liu, J. et al. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatology 70, 241–258 (2019).
Li, B. et al. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci. 197, 122–129 (2018).
Zhang, C. et al. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark. 21, 651–659 (2018).
Xu, H., Chen, Y., Dong, X. & Wang, X. Serum Exosomal Long Noncoding RNAs ENSG00000258332.1 and LINC00635 for the Diagnosis and Prognosis of Hepatocellular Carcinoma. Cancer Epidemiol. Biomarkers Prev. 27, 710–716 (2018).
Conigliaro, A. et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer 14, 155 (2015).
Li, X., Lei, Y., Wu, M. & Li, N. Regulation of Macrophage Activation and Polarization by HCC-Derived Exosomal lncRNA TUC339. Int. J. Mol. Sci. 19, 2958 (2019).
Takahashi, K., Yan, I. K., Wood, J., Haga, H. & Patel, T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 12, 1377–1387 (2014).
Zhang, H. et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene 38, 2844–2859 (2019).
Wang, G. et al. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a-MET pathway. EBioMedicine 40, 432–445 (2019).
Sergeeva, O., Sviridov, E. & Zatsepin, T. Noncoding RNA in Liver Regeneration-From Molecular Mechanisms to Clinical Implications. Semin. Liver Dis. 40, 70–83 (2020).
Song, X. J. et al. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci. 265, 118821 (2021).
Luo, L. et al. Exosomal MicroRNA-10a Is Associated with Liver Regeneration in Rats through Downregulation of EphA4. Chin. Med. J. (Engl) 131, 454–460 (2018).
Gebert, L. F. et al. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 42, 609–621 (2014).
Qu, Y. et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell Mol. Med. 21, 2491–2502 (2017).
Lou, G. et al. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell Mol. Med. 21, 2963–2973 (2017).
Lou, G. et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 8, 122 (2015).
Liu, Y. et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. Ebio Med. 36, 140–150 (2018).
Author information
Authors and Affiliations
Contributions
Z.Z.W. and Z.W. wrote the paper; X.Z., Y.C.S., Y.Q.Q., W.S.H., X.C.A., W.W.H., H.T.C., W.Q.Q. and Z.L.Y. revised the tables and figures; X.K.Y. and P.H.Y. deigned and revised the paper; all authors have read and approved the final version of this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval/consent to participate
All authors read and approved the final paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Z.W., Zheng, W., Xiang, Z. et al. Clinical implications of exosome-derived noncoding RNAs in liver. Lab Invest 102, 464–473 (2022). https://doi.org/10.1038/s41374-021-00723-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-021-00723-1
This article is cited by
-
MicroRNA regulation of AMPK in nonalcoholic fatty liver disease
Experimental & Molecular Medicine (2023)
-
Tumour-derived exosomal lncRNA SNHG16 induces telocytes to promote metastasis of hepatocellular carcinoma via the miR-942-3p/MMP9 axis
Cellular Oncology (2022)