Abstract
The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family includes nine members with aggrecan-degrading activity, i.e., ADAMTS1, 4, 5, 8, 9, 15, 16, 18, and 20. However, their systematic expression profile in knee osteoarthritis (OA) synovium and effects of cytokines and growth factors on the expression in OA synovial fibroblasts remain elusive. In this study, expression of all nine aggrecanolytic ADAMTS species was assessed by quantitative real-time PCR in OA and control normal synovial tissues. OA synovial fibroblasts were treated with interleukin-1α (IL-1α), IL-1β, tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), vascular endothelial growth factor165, and heparin-binding epidermal growth factor, and analyzed for the expression of the ADAMTS species. The signaling pathways and inhibition of ADAMTS4 expression by high-molecular-weight hyaluronan, adalimumab, tocilizumab, and signaling molecule inhibitors were studied. ADAMTS1, 4, 5, 9, and 16 were expressed in OA synovium, but only ADAMTS4 expression was significantly higher in OA as compared to normal synovium. IL-1α, TNF-α, and TGF-β markedly increased ADAMTS4 expression, while their effects were minimal for the other ADAMTS species. ADAMTS4 was synergistically upregulated by treatment with IL-1α and TNF-α, IL-1α and TGF-β, or IL-1α, TNF-α and TGF-β. The signaling molecules’ inhibitors demonstrated that IL-1α-induced ADAMTS4 expression is predominantly through TGF-β-associated kinase 1 (TAK1), and the TNF-α-stimulated expression is via TAK1 and nuclear factor-κB (NF-κB). The TGF-β-promoted expression was through the activin receptor-like kinase 5 (ALK5)/Smad2/3, TAK1, and non-TAK1 pathways. Adalimumab blocked TNF-α-stimulated expression. ADAMTS4 expression co-stimulated with IL-1α, TNF-α and TGF-β was abolished by treatment with adalimumab, TAK1 inhibitor, and ALK5/Smad2/3 inhibitor. These data demonstrate marked and synergistic upregulation of ADAMTS4 by IL-1α, TNF-α and TGF-β in OA synovial fibroblasts, and suggest that concurrent therapy with an anti-TNF-α drug and inhibitor(s) may be useful for prevention against aggrecan degradation in OA.
Similar content being viewed by others
Introduction
Osteoarthritis (OA), the most prevalent joint disease, is characterized by accelerated degradation of extracellular matrix (ECM) in the articular cartilage. The cartilage ECM consists mainly of hyaluronan-aggrecan networks and fibrils of types II and XI collagen. The initial histological change of the articular cartilage in the OA joint is depletion of the hyaluronan-aggrecan networks, and this change is followed by fibrillation and laceration of the cartilage due to degradation of collagen fibrils1. The “matrix metalloproteinase” (MMP) gene family members with collagenolytic activity such as MMP-1, MMP-13, and MMP-14 contribute to degradation of fibrillary collagens1, and members of the “a disintegrin and metalloproteinase with thrombospondin motifs” (ADAMTS) gene family play a central role in aggrecan degradation1,2,3. ADAMTS1, 4, 5, 8, 9, 15, 16, 18, and 20 show aggrecanolytic activity4,5,6, but among them, ADAMTS4 (aggrecanase-1) and ADAMTS5 (aggrecanase-2) may be major aggrecanases in OA because of their high specific activity to aggrecan and the expression by OA chondrocytes2,7. ADAMTS5 is believed to be essential to aggrecan degradation in mouse OA, since cartilage destruction in experimental OA models was suppressed in ADAMTS5-deficient mice, but not ADAMTS4-deficient mice8,9. However, an unanswered question is whether ADAMTS4 or ADAMTS5 is important in human OA2,7.
Degradation of the cartilage ECM in the OA joint was originally ascribed to the overexpression of these metalloproteinases by chondrocytes1. However, accumulating lines of evidence have suggested that local synovitis, which may be induced by synovial cell ingestion of debris released from damaged OA articular cartilage10, plays an important role in the OA progression by accelerating cartilage destruction11,12,13,14. Recent clinical studies have provided evidence that synovitis is an independent cause of OA15 and an independent driver of radiographic OA onset and progression16. In accordance with these clinical data, OA synovial fibroblasts and/or synovial lining cells are known to express ADAMTS417, ADAMTS518, MMP-119, and MMP-320, and the latter two enzymes are detected in synovial fluid from knee OA patients21. However, inconsistent data are reported about the expression of aggrecanolytic ADAMTS species in OA synovium: one study has shown that ADAMTS1, 4, 5, and 9 are downregulated in OA hip joints22, but other studies have demonstrated the overexpression of ADAMTS417 and ADAMTS518 in knee OA synovial cells. Thus, the expression profile of the aggrecanolytic ADAMTS species in knee OA synovial tissues requires additional investigation.
Although ADAMTS4 was reportedly upregulated by interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), or transforming growth factor-β (TGF-β) in synovial fibroblasts23, the expression of aggrecanolytic ADAMTS species after stimulation with these factors and their synergistic effects on ADAMTS4 expression have not been examined in OA synovial fibroblasts. In addition, little is known about the activation of ADAMTS4 intracellular signaling pathways after stimulation with these factors. High-molecular-weight HA (HMW-HA) is used for treatment of patients with knee OA as a symptom-modifying drug, and our study demonstrates that IL-1α-induced ADAMTS4 expression in OA chondrocytes is significantly reduced by HMW-HA treatment24. Biological agents targeting TNF-α or IL-6 receptor (IL-6R) have been introduced as disease-modifying anti-rheumatic drugs and are successful for the treatment of patients with rheumatoid arthritis25,26. However, effects of these anti-arthritic drugs on the cytokine(s)-stimulated ADAMTS4 expression in OA synovial fibroblasts were not studied.
In this study, we examined the systematic expression profile of all the nine aggrecanolytic ADAMTS species in synovial tissues from OA knee joints and studied the effects of cytokines and growth factors on their expression in OA synovial fibroblasts. Since ADAMTS4 expression was most markedly upregulated by IL-1, TNF-α, or TGF-β, synergistic effects were tested. We also investigated the intracellular signaling pathways and the inhibitory effects of HMW-HA, human anti-TNF-α antibody, and humanized anti-IL-6R antibody in OA synovial fibroblasts under stimulation with IL-1α, TNF-α, and/or TGF-β.
Materials and methods
Clinical samples
Synovial tissues were taken at total knee arthroplasty from knee OA patients diagnosed according to the criteria of the American College of Rheumatology27. Normal control synovial tissues were obtained at reconstruction surgery from knee joints of patients with anterior cruciate ligament injury. Synovial samples obtained from 15 OA patients and 6 patients with anterior cruciate ligament injury were used for examination of the mRNA expression of ADAMTS species, IL-1, TNF-α, and TGF-β1. OA synovial samples were also utilized for in vitro experiments by culturing synovial fibroblasts, and tissue samples were examined by histology and immunohistochemistry as described below. We obtained written informed consent from patients for experimental use of the samples. The study protocols that complied with the principles outlined in the Declaration of Helsinki were approved by the Keio University School of Medicine Ethics Committee (number 2012-046-4) and the Ethical Committee Review Board at Juntendo University (number 15-074).
RT-PCR and quantitative real-time PCR
OA and normal control synovial tissues removed at surgery were stored in RNA later solution (Thermo Fisher Scientific, Tokyo, Japan) at −20 °C, and total RNA was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany). cDNA was synthesized from 2 µg of total RNA using the ReverTraAce qPCR RT Master Mix (Toyobo, Osaka, Japan). For screening the mRNA expression of ADAMTS1, 4, 5, 8, 9, 15, 16, 18, and 20 in OA synovial tissue, cDNA was subjected to RT-PCR using primers listed in Supplementary Table S1, which is available at the Laboratory Investigation website. Positive control cells used for the ADAMTS species were as follows: synovial fibroblasts isolated from rheumatoid arthritis patients for ADAMTS1, ADAMTS4, and ADAMTS5; CaR-1 colon carcinoma cell line (JCRB Cell Bank, Osaka, Japan) for ADAMTS8; U251 glioblastoma cell line (JCRB Cell Bank, Osaka, Japan) for ADAMTS9 and ADAMTS15; PC-9 lung adenocarcinoma cell line (Immuno-Biological Laboratories, Gumma, Japan) for ADAMTS16 and ADAMTS20; human umbilical vein endothelial cells (Cambrex, East Rutherford, NJ, USA) for ADAMTS18. ADAMTS1, 4, 5, 9, and 16 were detected in OA synovial tissue (Fig. 1A), and their mRNA expression levels relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using THUNDERBIRD SYBR qPCR Mix (TOYOBO, QPS-201) according to the ΔΔCT method28 were examined by quantitative real-time PCR in OA (n = 15) and normal control (n = 6) synovial tissues. The mRNA expression levels of IL-1α, IL-1β, TNF-α, and TGF-β1 in OA (n = 14) and control (n = 5) synovial tissues were also measured by a similar quantitative real-time PCR. The primers for ADAMTS1, 4, 5, 9, 16, IL-1α, IL-1β, TNF-α, TGF-β1, and GAPDH are described in Supplementary Table S2, which is available at the Laboratory Investigation website. The mean ratios of ADAMTS species, IL-1α, IL-1β, TNF-α, or TGF-β1 to GAPDH in normal control samples were set at 1.
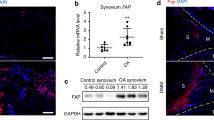
A RT-PCR analysis of the mRNA expression of ADAMTS1, 4, 5, 8, 9, 15, 16, 18, and 20 in OA synovial tissues from knee OA patients (n = 9), showing the positive expression of ADAMTS1, 4, 5, 9, and 16. PC, positive control for each ADAMTS species. B Quantitative real-time PCR analysis of ADAMTS1, 4, 5, 9, and 16 expression in normal control (NOR; n = 6) and OA (n = 15) synovial tissues. The mean ADAMTS species:GAPDH ratio in normal control samples is set at 1. Circles, individual subjects; horizontal lines, means. *p < 0.05 by Mann–Whitney U test.
The expression levels of the ADAMTS species in OA synovial fibroblasts, which were treated with cytokines or growth factors as described below, were also measured by the above quantitative real-time PCR method28. Similarly, we examined the mRNA expression levels of MMP-1, MMP-3, and MMP-13 in OA synovial fibroblasts under stimulation with IL-1α, TNF-α, and/or TGF-β by the same quantitative real-time PCR method using the primers described in Supplementary Table S2.
Histology, immunohistochemistry, and antibodies against ADAMTS4
OA synovial tissues (n = 3) were fixed in buffered formalin, embedded in paraffin, sectioned in 4-µm thick and stained with hematoxylin and eosin. For immunohistochemistry, the sections were treated with 0.3% H2O2 and 10% normal goat serum to block endogenous peroxidase and non-specific reactions, and then reacted with mouse anti-ADAMTS4 monoclonal antibody (10 µg/ml; 247-3F6) or mouse non-immune IgG (10 µg/ml; Abcam, Cambridge, UK)24,29. In our previous study, two mouse monoclonal antibodies specific to the spacer domain (247-3F6) or a portion of the disintegrin and thrombospondin domains (250-4F7) of human ADAMTS4 were developed29. Specificity of these antibodies, which can be used for immunoblotting, immunoprecipitation, and immunohistochemistry, has been previously demonstrated24,29. They were reacted with goat anti-mouse IgG conjugated to peroxidase-labeled dextran polymer (no dilution; En Vision+ mouse; Dako, Glostrup, Denmark) and color was developed with 3,3’-diaminobenzidine tetrahydrochloride in 50 mM Tris-HCl buffer, pH 7.6 containing 0.006% H2O2. Counterstaining was performed with hematoxylin.
Cell cultures
Fresh OA synovial tissues were obtained at knee arthroplasty from a total of 17 patients with knee OA, and primary synovial fibroblasts were isolated by incubating the minced tissue fragments at 37 °C for 3 h with a mixture of 0.4% bacterial collagenase (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan) and 50 µg/ml DNase (Sigma-Aldrich, St. Louis, MO, USA) in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific) containing 10% fetal bovine serum and penicillin/streptomycin by modification of our previous methods30. Isolated cells were seeded in culture dishes and maintained in the serum-containing DMEM in a CO2 incubator. After starvation in serum-free DMEM containing 0.2% lactalbumin hydrolysate (Sigma-Aldrich), synovial fibroblasts at passages 2–3 were treated for 24 h with IL-1α (R&D Systems, Minneapolis, MN, USA), IL-1β (R&D Systems), TNF-α (R&D Systems), TGF-β (R&D Systems), vascular endothelial growth factor165 (VEGF; R&D Systems), or heparin-binding epidermal growth factor (HB-EGF; R&D Systems) in the same medium, and subjected to mRNA expression analysis (see below). Where indicated, they were also treated with IL-1α, TNF-α, and/or TGF-β.
For inhibition studies, serum-starved OA synovial fibroblasts were treated for 1 h (or 24 h for HMW-HA treatment) with inhibitors such as TGF-β-associated kinase 1 (TAK1) inhibitor (5Z-7-Oxozeaenol; Sigma-Aldrich), nuclear factor-κB (NF-κB) inhibitor (BAY11-7082; Fujifilm Wako Pure Chemical Corporation, Osaka, Japan), activin receptor-like kinase 5 (ALK5) inhibitor (SB505124; Sigma-Aldrich), and ALK1 inhibitor (LDN193189; Sigma-Aldrich) and/or clinically used drugs for arthritis including HMW-HA (Suvenyl®; Chugai Pharmaceutical Co., LTD, Tokyo, Japan), adalimumab (human anti-TNF-α antibody; Humira®, AbbVie Inc., North Chicago, IL, USA), and tocilizumab (humanized anti-IL-6R antibody; Actemra®, Chugai Pharmaceutical Co., LTD). For the synergistic studies, the cultures were then treated with IL-1α, TNF-α, and/or TGF-β in DMEM containing 0.2% lactalbumin hydrolysate.
Immunoblotting of ADAMTS4
Serum-starved OA synovial fibroblasts were treated with IL-1α, TNF-α, and/or TGF-β in DMEM containing 0.2% lactalbumin hydrolysate for 72 h. After washing in phosphate buffered saline, they were incubated with 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer containing 2-mercaptoethanol and cell lysates were sonicated on ice. Supernatants were obtained by centrifugation and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on NuPAGE 4-12% Bis-Tris gels (InvitroGen, Carlsbad, CA, USA), and the proteins resolved were transferred onto PVDF membranes. After blocking non-specific reactions with 5% skim milk in phosphate buffered saline, the membranes were reacted with mouse anti-ADAMTS4 monoclonal antibody (0.5 µg/ml; 250-4F7) or anti-GAPDH antibody (1:1000 dilution; Abcam, Cambridge, UK)24,29, followed by incubation with horseradish peroxidase-conjugated secondary antibodies and reaction with ECL western blotting reagents (GE Healthcare Bio-Sciences, Uppsala, Sweden). The proteins were detected by chemiluminescence imaging system using Amersham Imager 680 (GE Healthcare Life Sciences, Tokyo, Japan) and intensity of the bands was quantified by densitometric analysis using ImageJ software (http://rsb.info.nih.gov/ij/).
For immunoblotting of ADAMTS4 in OA synovial tissue, supernatants (50 µg/lane) of the tissue homogenates (n = 3) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins transferred on PVDF membranes were incubated with anti-ADAMTS4 antibodies (0.5 µg/ml each; 247-3F6 or 250-4F7)24,29 or anti-GAPDH antibody (1:1000 dilution; Abcam), followed by the reactions with secondary antibody and ECL reagents as described above.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA) (www.graphpad.com). Comparisons involving more than three groups were analyzed using the Kruskal–Wallis test. When p < 0.05, then pairwise comparisons were made using the Mann–Whitney U test. The results are presented as means ± SD, and p values less than 0.05 were considered statistically significant.
Results
Expression of aggrecanolytic ADAMTS species and protein expression of ADAMTS4 in OA synovial tissue
mRNA expression of all nine ADAMTS species with aggrecanolytic activity including ADAMTS1, 4, 5, 8, 9, 15, 16, 18, and 20 was screened by RT-PCR in synovial tissue from knee OA patients (n = 9). As shown in Fig. 1A, expression of ADAMTS1, 4, 5, 9, and 16 was observed in OA synovium, whereas there was negligible expression of ADAMTS8, 15, 18, and 20. Thus, we further analyzed the expression of ADAMTS1, 4, 5, 9, and 16 in a larger number of OA synovial tissue (n = 15) and normal control synovial tissue (n = 6) by quantitative real-time PCR. The expression levels of these ADAMTS species were compared by setting the average level in the control synovial tissue at 1. Only ADAMTS4 expression was significantly higher in OA synovial tissue (3.70 ± 3.79; mean ± SD) than in the control samples (1.00 ± 0.84) (p = 0.019) (Fig. 1B). The expression levels of ADAMTS1 (3.30 ± 3.44) and ADAMTS16 (1.96 ± 1.17) in OA synovial tissue samples appeared to be higher than in the control samples (1.00 ± 0.53 and 1.00 ± 0.49 for ADAMTS1 and ADAMTS16, respectively), but no significant difference was obtained between the two groups (p = 0.052 and p = 0.087, respectively) (Fig. 1B). The expression levels of ADAMTS5 in the OA and control synovial samples were almost similar (1.18 ± 0.46 versus 1.00 ± 0.54) and the levels of ADAMTS9 (0.56 ± 0.49) in the OA samples were lower than those in the control samples (1.00 ± 0.62), although not significantly different (Fig. 1B).
OA synovium showed hyperplasia of the synovial lining cells and lymphoid cell infiltration in the sub-lining cell layer (Supplementary Fig. S1A), both of which were only mild degree as compared to synovial tissue obtained from patients with rheumatoid arthritis30. By immunohistochemistry, ADAMTS4 was localized mainly to hyperplastic synovial lining cells, while immunostaining with non-immune IgG showed only background staining (Supplementary Fig. S1A). Immunoblotting analysis indicated that OA synovial tissue contains a 58-kDa protein band of ADAMTS4 as a major band, which corresponds to an NH2- and COOH-terminal truncated active form of ADAMTS429, together with some smaller fragments (Supplementary Fig. S1B).
Expression of aggrecanolytic ADAMTS species in OA synovial fibroblasts upon stimulation by cytokines and growth factors
Inflammatory cytokines and growth factors including IL-1α, IL-1β, TNF-α, TGF-β, VEGF, and HB-EGF are known to be expressed in OA joints31,32. In fact, our quantitative real-time PCR analysis demonstrated that the mRNA expression levels of IL-1α, IL-1β, TNF-α, and TGF-β are significantly higher in OA synovial tissue (n = 14) than normal control synovial tissue (n = 5) (Supplementary Fig. S2). Therefore, we examined the effects of these factors on the expression of all the nine ADAMTS species with aggrecanase activity4,5,6. As shown in Fig. 2A (left panel), RT-PCR showed that OA synovial fibroblasts under unstimulated conditions have a similar expression profile of ADAMTS species as that of OA synovium. IL-1α, IL-1β, TNF-α, and TGF-β stimulated ADAMTS4 expression, but no or negligible effect was observed on ADAMTS4 expression by VEGF or HB-EGF (Fig. 2A). The expression of ADAMTS1, ADAMTS9, and ADAMTS16 also seemed to be slightly altered by treatment with IL-1α, IL-1β, TNF-α, and TGF-β (Fig. 2A). Conversely, ADAMTS5 expression appeared to be constitutive without a change in expression induced by these factors (Fig. 2A). No induction for ADAMTS8, 15, 18, or 20 was seen by RT-PCR even after treatment with these factors (Fig. 2A).
A RT-PCR analysis of the mRNA expression of ADAMTS1, 4, 5, 8, 9, 15, 16, 18, and 20 in OA synovial fibroblasts treated with IL-1α, IL-1β, TNF-α, TGF-β, VEGF, or HB-EGF. OA synovial fibroblasts were treated with three different concentrations of these factors for 24 h and changes in the mRNA expression were analyzed by RT-PCR. Control represents cultures without the factors. The experiments were carried out in triplicate, and representative data are shown. B Quantitative real-time PCR analysis of the ADAMTS species expression in OA synovial fibroblasts treated with IL-1α, TNF-α, TGF-β, VEGF, or HB-EGF. OA synovial fibroblasts were stimulated with IL-1α (10 ng/ml), TNF-α (10 ng/ml), TGF-β (10 ng/ml), VEGF (50 ng/ml), or HB-EGF (120 ng/ml) for 24 h. The mean ADAMTS species:GAPDH ratio in control samples without cytokine/growth factor treatment is set at 1. *p < 0.05; **p < 0.01 by Mann–Whitney U test.
Quantitative real-time PCR demonstrated that ADAMTS4 expression is upregulated 55-fold by IL-1α, 16-fold by TNF-α, or 27-fold by TGF-β, but no significant changes were observed by treatment with VEGF or HB-EGF (Fig. 2B). These cytokines also significantly (6- to 8-fold) increased ADAMTS16 expression. The expression of ADAMTS5 was significantly 5-fold increased by IL-1α (Fig. 2B). Only negligible or insignificant changes were seen in the expression levels of ADAMTS1, 8, 9, 15, 18, or 20 under stimulation with these factors (Fig. 2B). Since the expression of ADAMTS4 was significantly higher in OA synovial tissue samples as compared to the control samples (Fig. 1B) and markedly upregulated by IL-1α, TNF-α, or TGF-β (Fig. 2B), we further examined the synergistic effects of these cytokines by focusing on ADAMTS4 expression.
Synergistic effects of IL-1α, TNF-α, and TGF-β on the expression of ADAMTS4 and MMPs in OA synovial fibroblasts
The mRNA expression of ADAMTS4 was significantly upregulated by combined treatment with IL-1α and TNF-α, or IL-1α and TGF-β, but no synergy was obtained by treatment with TNF-α and TGF-β (Fig. 3A). The synergistic effect on mRNA expression was also obtained by treatment with IL-1α, TNF-α and TGF-β (Fig. 3A). In accordance with enhanced mRNA expression, densitometric analysis of immunoblotting data demonstrated synergistic stimulation of the ADAMTS4 protein expression by combined treatments with IL-1α and TNF-α, IL-1α and TGF-β, or IL-1α, TNF-α and TGF-β (Fig. 3B). Stimulation with these factors increased density of the 73-kDa protein band, which corresponds to an active form of ADAMTS424,29,33. The 100-kDa zymogen form of ADAMTS433 commonly appeared when synovial fibroblasts were treated with TNF-α or when the 73-kDa form was over-produced by combined treatment with these factors (Fig. 3B).
A Quantitative real-time PCR analysis of ADAMTS4 mRNA expression in OA synovial fibroblasts treated with IL-1α, TNF-α, and/or TGF-β. OA synovial fibroblasts were stimulated with IL-1α (10 ng/ml), TNF-α (10 ng/ml), and/or TGF-β (10 ng/ml) for 24 h. The average ADAMTS4:GAPDH ratio in the cells without treatment was set at 1. B Immunoblotting analysis of the ADAMTS4 protein expression in OA synovial fibroblasts treated with IL-1α, TNF-α, and/or TGF-β. OA synovial fibroblasts were treated with IL-1α, TNF-α, and/or TGF-β for 72 h as described above, and cell lysates were subjected to immunoblotting analysis. Arrowhead and arrow, 100-kDa zymogen and 73-kDa active forms of ADAMTS4. Ratios of ADAMTS4 to GAPDH were quantified by densitometry of immunoblotted bands. Values are means ± SD (n = 5 each group). *p < 0.05; **p < 0.01 by Mann–Whitney U test.
We then examined effects of IL-1α, TNF-α, and TGF-β on the mRNA expression of MMP-1, MMP-3, and MMP-13, and compared the data between ADAMTS4 and these MMPs. As shown in Supplementary Fig. S3, IL-1α significantly upregulated the expression of MMP-1, MMP-3, and MMP-13, whereas TNF-α stimulated only the expression of MMP-1. On the other hand, TGF-β effect was complex: TGF-β downregulated the MMP-1 expression and showed no effect on the MMP-3 expression, although it stimulated the MMP-13 expression. Importantly, MMP-1 and MMP-3 expression was upregulated by the combined treatment of IL-1α and TNF-α but was significantly suppressed by TGF-β treatment (Supplementary Fig. S3). In addition, no synergistic upregulation of the MMP-1, MMP-3, and MMP-13 expression was observed by combined treatment with TNF-α, IL-1α, and TGF-β (Supplementary Fig. S3). These data were different from the synergistic effect of IL-1α, TNF-α, and TGF-β on ADAMTS4 expression (Fig. 3A).
Effects of inhibitors to TAK1, NF-κB, ALK1, and ALK5 on the ADAMTS4 expression in cytokine-stimulated synovial fibroblasts
Mitogen-activated protein kinases (MAPKs) and/or NF-κB are known to be the second messengers for ADAMTS4 expression in various cells stimulated with IL-1, TNF-α, or TGF-β34,35,36,37, and TAK1 plays a key role as the main upstream molecule for the MAPK and NF-κB pathways38. Thus, we investigated the involvement of TAK1 in ADAMTS4 expression, and found that the ADAMTS4 expression stimulated by IL-1α is almost completely blocked with TAK1 inhibitor (6.94 ± 3.84% of the original expression level) (Fig. 4A), indicating that the IL-1α-stimulated ADAMTS4 expression is mediated through TAK1. The TNF-α-stimulated ADAMTS4 expression was partially inhibited by TAK1 inhibitor (23.97 ± 12.58%) or NF-κB inhibitor (BAY11-7082) (54.11 ± 5.59%), and almost completely suppressed by combined treatment with TAK1 inhibitor and NF-κB inhibitor (10.58 ± 3.15%) (Fig. 4B). The TGF-β-induced ADAMTS4 expression was partially inhibited by TAK1 inhibitor (62.39 ± 10.23%) (Fig. 4C). However, ALK5 inhibitor (SB505124) completely abrogated the TGF-β-induced ADAMTS4 expression (1.50 ± 0.75%), whereas ALK1 inhibitor (LDN193189) did not show inhibition (90.40 ± 24.59%) (Fig. 4C).
A Effect of 1 µM TAK1 inhibitor (TAK1i) on IL-1α-induced ADAMTS4 expression. B 1 µM TAK1 inhibitor and/or 5 µM NF-κB inhibitor (BAY11-7082) on TNF-α-induced ADAMTS4 expression. C 1 µM TAK1 inhibitor, 5 µM ALK5 inhibitor (SB505124), or 1 µM ALK1 (LDN193189) on TGF-β-induced ADAMTS4 expression. Cells were treated with inhibitors for 1 h prior to stimulation with the factors (10 ng/ml each) for 72 h. D–F Effects of HMW-HA, adalimumab or tocilizumab on IL-1α, TNF-α, or TGF-β-induced ADAMTS4 expression. OA synovial fibroblasts were treated with 2.5 mg/ml HMW-HA for 24 h, or 10 µg/ml adalimumab or 10 µg/ml tocilizumab for 1 h prior to stimulation with 10 ng/ml IL-1α (D), 10 ng/ml TNF-α (E), or 10 ng/ml TGF-β (F) for 72 h. Ratios of ADAMTS4 to GAPDH were quantified by densitometry of immunoblotted bands. Values are means ± SD (n = 5 each group). **p < 0.01 by Mann–Whitney U test.
Effects of HMW-HA, anti-TNF-α antibody, and anti-IL-6R antibody on the ADAMTS4 expression in cytokine-stimulated synovial fibroblasts
HMW-HA is clinically used as a symptom-modifying treatment for patients with knee OA, and adalimumab (anti-TNF-α antibody) and tocilizumab (anti-IL-6R antibody) are biologic medications, which are commonly used for treatment of patients with rheumatoid arthritis. We therefore tested these drugs on IL-1α-, TNF-α-, or TGF-β-stimulated ADAMTS4 expression in OA synovial fibroblasts. As shown in Fig. 4D, E, HMW-HA significantly inhibited IL-1α or TNF-α-stimulated ADAMTS4 expression (76.38 ± 12.38% and 65.70 ± 18.24%, respectively). Adalimumab significantly inhibited IL-1α-stimulated ADAMTS4 expression (79.83 ± 10.71%) (Fig. 4D), and as expected, completely abrogated the TNF-α-induced ADAMTS4 expression (0.14 ± 0.08%) (Fig. 4E). However, negligible inhibition of the TGF-β-stimulated ADAMTS4 expression was found by treatment with these drugs (Fig. 4F).
Effects of inhibitors to signaling molecules and anti-arthritic drugs on the ADAMTS4 expression stimulated with IL-1α, TNF-α, and TGF-β
Since TAK1 inhibitor, ALK5 inhibitor (SB505124) or NF-κB inhibitor (BAY11-7082) effectively suppressed IL-1α-, TNF-α-, or TGF-β-stimulated ADAMTS4 expression (see Fig. 4), we then tested the effects of these inhibitors on the three cytokine (IL-1α, TNF-α, and TGF-β)-stimulated ADAMTS4 expression. Figure 5A shows that TAK1, ALK5, and NF-κB inhibitors significantly reduced ADAMTS4 expression (32.70 ± 8.53%, 42.65 ± 7.64%, and 79.38 ± 11.05%, respectively). When the inhibitory effects of HMW-HA, adalimumab, and tocilizumab on the three cytokine-stimulated ADAMTS4 expression were examined, only partial inhibition was obtained by treatment with HMW-HA (76.83 ± 7.21%), adalimumab (62.34 ± 12.39%), tocilizumab (87.23 ± 3.91%), or all three together (68.43 ± 9.33%) (Fig. 5B).
A 1 µM TAK1 inhibitor (TAK1i), 5 µM ALK5 inhibitor (SB505124), or 5 µM NF-κB inhibitor (BAY11-7082). B 2.5 mg/ml HMW-HA, 10 µg/ml adalimumab, and/or 10 µg/ml tocilizumab. C Combined effect of the three drugs (HMW-HA, adalimumab, and tocilizumab) and TAK1 inhibitor and/or NF-κB inhibitor. D Combined effect of adalimumab and TAK1 inhibitor and/or ALK5 inhibitor. OA synovial fibroblasts were treated with inhibitors and/or drugs prior to stimulation with IL-1α, TNF-α, and TGF-β for 72 h. Values are means ± SD (n = 5 each group). **p < 0.01 by Mann–Whitney U test.
We further explored the conditions of complete inhibition of the three cytokine-stimulated ADAMTS4 expression by treatment with three anti-arthritic drugs together with TAK1 inhibitor and/or ALK5 inhibitor (SB505124). As shown in Fig. 5C, the expression levels were decreased remarkably by the combined treatment with either TAK1 inhibitor or ALK5 inhibitor (10.54 ± 4.40% and 11.68 ± 2.73%, respectively), and completely inhibited by combination of all these drugs and inhibitors (0.95 ± 0.84%). Since adalimumab was the most effective for TNF-α-stimulated or the three cytokine-stimulated ADAMTS4 expression among the three drugs, we also examined the inhibitory effects of adalimumab and TAK1 inhibitor and/or ALK5 inhibitor. The ADAMTS4 expression was significantly reduced by the combined treatment with adalimumab and TAK1 inhibitor or ALK5 inhibitor (21.76 ± 3.94% and 16.79 ± 2.92%, respectively) (Fig. 5D). A similar level of inhibition (11.11 ± 1.46%) was obtained by treatment with TAK1 and ALK5 inhibitors, and almost complete inhibition was obtained by combined treatment with adalimumab, TAK1 inhibitor, and ALK5 inhibitor (3.27 ± 0.49%) (Fig. 5D).
Discussion
The present study is, to the best of our knowledge, the first to demonstrate that among all the nine aggrecanolytic ADAMTS species, ADAMTS1, 4, 5, 9, and 16 are expressed in both synovium and synovial fibroblasts from OA knee joints. Our previous studies indicated that OA cartilage and/or chondrocytes express ADAMTS1, 4, 5, 9, and 15 among the six aggrecanolytic ADAMTS species examined (i.e., ADAMTS1, 4, 5, 8, 9, and 15)24,29, and similar results were reported in OUMS-27 chondrosarcoma cells39. Since a previous study has shown that OA cartilage expresses ADAMTS1640, OA synovial fibroblasts and chondrocytes appear to have a similar expression profile except for ADAMTS15, which was expressed by OA chondrocytes, but not synovial fibroblasts.
ADAMTS4 and ADAMTS5 have strong aggrecanase activity compared with ADAMTS1, 8, 9, 15, 16, 18, and 202,5,7. In conjunction with this biochemical property, tissue localization in human OA cartilage, and loss of function in knockout mice, both ADAMTS4 and ADAMTS5 expressed by chondrocytes are suggested to be critically important as aggrecanase in OA cartilage2,7. On the other hand, emerging evidence has indicated the involvement of synovitis in OA progression by accelerating cartilage destruction11,12,13,14, and recent studies on knee OA have suggested that synovitis is an independent driver of radiographic OA onset and progression15,16. In the present study, we have demonstrated that among the five ADAMTS species expressed in the OA synovium, only ADAMTS4 is significantly overexpressed in OA synovium compared to normal synovium, and markedly upregulated by treatment with IL-1α, TNF-α, or TGF-β, whereas ADAMTS5 expression is constitutive in the synovium even under stimulation. In addition, like ADAMTS4 from OA chondrocytes under culture24 or within cartilage tissue29, ADAMTS4 was detected as a 73-kDa active form in cultured OA synovial fibroblasts, activity of which can be inhibited by binding to fibronectin, but in OA synovium, it existed as a 58-kDa truncated form, which is fully active secreted form without being trapped by the ECM33. Thus, all these data suggest that ADAMTS4 overexpressed by stimulation with IL-1α, TNF-α, and/or TGF-β in synovium and secreted to synovial fluid may contribute to accelerated cartilage damage through aggrecan degradation in the superficial areas of OA articular cartilage.
Previous studies showed that ADAMTS4 expression is promoted by IL-124,34,41, TNF-α41, TGF-β23,41,42, or oncostatin M43 in various types of interstitial cells, and synergistic effects on the expression were reported with IL-1 and TNF-α in rat nucleus pulposus cells35, IL-1 and TGF-β in human tendon cells41, and IL-1 and oncostatin M in human chondrocytes43. This study provides the first evidence that OA synovial fibroblasts synergistically upregulate ADAMTS4 expression by treatment with IL-1α and TNF-α, IL-1α and TGF-β, and also IL-1α, TNF-α and TGF-β. Since IL-1 and TNF-α are catabolic pro-inflammatory cytokines and they synergistically promote the expression of ECM-degrading metalloproteinases such as MMP-1 and MMP-344, synergistic effect of these cytokines could be expected for the ADAMTS4 expression in OA synovial fibroblasts. However, TGF-β has more complex effects than IL-1 and TNF-α. TGF-β is reported to counteract the catabolic effects of TNF-α and IL-1 by downregulation of collagenolytic MMPs such as MMP-13 and MMP-1445,46, and the present study has shown the counteractive activity of TGF-β on the expression of MMP-1 and MMP-3, both of which were upregulated by IL-1α and/or TNF-α. The absence of a synergistic effect on ADAMTS4 expression by TGF-β and TNF-α may be explained by the fact that TNF-α has an antagonistic effect on TGF-β signaling pathways in skin fibroblasts47. The mechanism on the synergistic effects of TGF-β with IL-1α and TNF-α on the ADAMTS4 expression remains unclear in the present study. However, a previous study on the analysis of genome-wide gene expression in chondrocytes indicated that IL-1 and TGF-β have additive effect on the expression of some genes including VEGF and connective tissue growth factor46, both of which are induced by TGF-β46,48, like ADAMTS423. In addition, cross-talk between IL-1 and TGF-β has been demonstrated49. Therefore, it seems likely that the expression of TGF-β-inducible genes such as ADAMTS4 and VEGF could be stimulated by IL-1 and TNF-α, although further studies are needed to clarify the precise molecular mechanism.
TAK1 is a key player in the cascade of cellular responses evoked by various cytokines including IL-1α, TNF-α, and TGF-β38. This study demonstrated that TAK1 inhibition significantly suppresses ADAMTS4 expression induced by these three factors, but inhibition rates were different depending on the factors used. For example, the IL-1α-stimulated ADAMTS4 expression was almost completely inhibited by TAK1 inhibitor (Fig. 4A), indicating that IL-1α signaling is predominantly through TAK1 (Fig. 6). Partial inhibition of the TNF-α-induced ADAMTS4 expression by either TAK1 inhibitor or NF-κB inhibitor and almost complete inhibition by their combined treatment (Fig. 4B) suggest that the expression under stimulation with TNF-α is dependent on the TAK1 pathway and partially, but directly without passing through TAK1, on the NF-κB pathway (Fig. 6). Similarly, since TAK1 inhibitor suppressed ~40% of the ADAMTS4 expression (Fig. 4C), the TGF-β-induced ADAMTS4 expression may be through both TAK1 and non-TAK1 pathways (Fig. 6). Accumulated lines of evidence on OA articular cartilage have indicated that TGF-β transduces antagonistic signals by activating the Smad2/3 and Smad1/5/8 pathways through the different TGF-β type I receptors, i.e., ALK5 and ALK1, respectively, and TGF-β signaling through the ALK1 and Smad1/5/8 pathway is favored in OA chondrocytes50. In OA synovial fibroblasts, however, the ALK5 and Smad2/3 pathway is considered a main pathway for ADAMTS4 expression (Fig. 6), since the TGF-β-induced expression was completely inhibited by treatment with ALK5 inhibitor, but not ALK1 inhibitor (Fig. 4C).
Because of the almost complete inhibition mediated by TAK1 inhibitor, IL-1α-induced ADAMTS4 expression is through the TAK1 pathway. The data of ~70% inhibition by TAK1 inhibitor and ~50% inhibition by NF-κB inhibitor demonstrate that TNF-α-stimulated ADAMTS4 expression is mainly through the TAK1 pathway and partially, but directly, via the NF-κB pathway. Complete inhibition by ALK5 inhibitor indicates that TGF-β-stimulated ADAMTS4 expression is caused through the ALK5 and Smad2/3 pathway, under which the signal goes through the TAK1 pathway and the non-TAK1 pathway.
Our previous study demonstrated that IL-1α-induced ADAMTS4 expression in OA chondrocytes is markedly reduced by HMW-HA treatment24. In the present study, we found that HMW-HA downregulates the ADAMTS4 expression induced by IL-1α or TNF-α in OA synovial fibroblasts. Since adalimumab partially suppressed IL-1α-induced ADAMTS4 expression, TNF-α is implicated, at least in part, in the ADAMTS4 expression promoted by IL-1α, which is known to induce TNF-α51. The data that the ADAMTS4 expression induced by combined treatment with IL-1α, TNF-α and TGF-β was only partially inhibited by HMW-HA, adalimumab and/or tocilizumab suggest that none of the drugs alone is useful for sufficient inhibition of the ADAMTS4 expression in OA patients. Inhibitors to TAK152,53 or ALK554,55 have been recently developed to block these signaling pathways with the intention to treat patients with cancers or immunological diseases such as rheumatoid arthritis. Since combined treatment of anti-arthritic drugs (HMW-HA, adalimumab, and tocilizumab) or adalimumab alone with inhibitors of TAK1 and ALK5 abolished ADAMTS4 expression induced by IL-1α, TNF-α and TGF-β, our data on the signaling pathways and the effects of TAK1 and ALK5 inhibitors provide a framework for the development of future concurrent therapies with ready-made drugs such as adalimumab to protect the aggrecan degradation in OA cartilage.
Data availability
The data used to support the findings of this study are included within the article, and Supplementary data are available at Laboratory Investigation online.
References
Okada, Y. Proteinases and matrix degradation. In Kelley and Firestein’s Textbook of Rheumatology 10th edn (eds Firestein, G. S., Budd, R. C., Gabriel, S. E., McInnes, I. B. & O’Dell, J. R.) 106–125 (Elsevier Inc., 2017).
Fosang, A. J. & Little, C. B. Drug insight: aggrecanases as therapeutic targets for osteoarthritis. Nat. Clin. Pract. Rheumatol. 4, 420–427 (2008).
Shiomi, T., Lemaitre, V., D’Armiento, J. & Okada, Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol. Int. 60, 477–496 (2010).
Porter, S., Clark, I. M., Kevorkian, L. & Edwards, D. R. The ADAMTS metalloproteinases. Biochem. J. 386, 15–27 (2005).
Jones, G. C. & Riley, G. P. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res. Ther. 7, 160–169 (2005).
Zeng, W. et al. Glycosaminoglycan-binding properties and aggrecanase activities of truncated ADAMTSs: comparative analyses with ADAMTS-5, -9, -16 and -18. Biochim. Biophys. Acta 1760, 517–524 (2006).
Tortorella, M. D. & Malfait, A. M. Will the real aggrecanase(s) step up: evaluating the criteria that define aggrecanase activity in osteoarthritis. Curr. Pharm. Biotechnol. 9, 16–23 (2008).
Glasson, S. S. et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434, 644–648 (2005).
Stanton, H. et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434, 648–652 (2005).
Lindblad, S. & Hedfors, E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 30, 1081–1088 (1987).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature Rev. Rheumatol. 6, 625–635 (2010).
Ayral, X., Pickering, E. H., Woodworth, T. G., Mackillop, N. & Dougados, M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13, 361–367 (2005).
Felson, D. T. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage 21, 10–15 (2013).
MacFarlane, L. A. et al. Association of changes in effusion-synovitis with progression of cartilage damage over eighteen months in patients with osteoarthritis and meniscal tear. Arthritis Rheumatol. 71, 73–81 (2019).
Felson, D. T. et al. Synovitis and the risk of knee osteoarthritis: the MOST Study. Osteoarthritis Cartilage 24, 458–464 (2016).
Mathiessen, A. & Conaghan, P. G. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res. Ther. 19, 18 (2017).
Perez-Garcia, S. et al. Healthy and osteoarthritic synovial fibroblasts produce a disintegrin and metalloproteinase with thrombospondin motifs 4, 5, 7, and 12: Induction by IL-1beta and fibronectin and contribution to cartilage damage. Am. J. Pathol. 186, 2449–2461 (2016).
Vankemmelbeke, M. N. et al. Expression and activity of ADAMTS-5 in synovium. Eur. J. Biochem. 268, 1259–1268 (2001).
Wyatt, L. A. et al. Molecular expression patterns in the synovium and their association with advanced symptomatic knee osteoarthritis. Osteoarthritis Cartilage 27, 667–675 (2019).
Okada, Y. et al. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab. Invest. 66, 680–690 (1992).
Yoshihara, Y. et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann. Rheum. Dis. 59, 455–461 (2000).
Davidson, R. K. et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res. Ther. 8, R124 (2006).
Yamanishi, Y. et al. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J. Immunol. 168, 1405–1412 (2002).
Yatabe, T. et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann. Rheum. Dis. 68, 1051–1058 (2009).
Lovell, D. J. et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N. Engl J. Med. 359, 810–820 (2008).
De Benedetti, F. et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl J. Med. 367, 2385–2395 (2012).
Altman, R. et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 29, 1039–1049 (1986).
Chijiiwa, M. et al. CCN1 (Cyr61) is overexpressed in human osteoarthritic cartilage and inhibits ADAMTS-4 (aggrecanase 1) activity. Arthritis Rheumatol. 67, 1557–1567 (2015).
Naito, S. et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol. Int. 57, 703–711 (2007).
Takizawa, M. et al. Production of tissue inhibitor of metalloproteinases 3 is selectively enhanced by calcium pentosan polysulfate in human rheumatoid synovial fibroblasts. Arthritis Rheum. 43, 812–820 (2000).
Boehme, K. A. & Rolauffs, B. Onset and progression of human osteoarthritis—can growth factors, inflammatory cytokines, or differential miRNA expression concomitantly induce proliferation, ECM degradation, and inflammation in articular cartilage? Int. J. Mol. Sci. 19, 2282 (2018).
Long, D. L., Ulici, V., Chubinskaya, S. & Loeser, R. F. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is increased in osteoarthritis and regulates chondrocyte catabolic and anabolic activities. Osteoarthritis Cartilage 23, 1523–1531 (2015).
Hashimoto, G., Shimoda, M. & Okada, Y. ADAMTS4 (aggrecanase-1) interaction with the C-terminal domain of fibronectin inhibits proteolysis of aggrecan. J. Biol. Chem. 279, 32483–32491 (2004).
Ahmad, R., Sylvester, J., Ahmad, M. & Zafarullah, M. Adaptor proteins and Ras synergistically regulate IL-1-induced ADAMTS-4 expression in human chondrocytes. J. Immunol. 182, 5081–5087 (2009).
Tian, Y. et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am. J. Pathol. 182, 2310–2321 (2013).
Kataoka, Y. et al. Mechanisms involved in suppression of ADAMTS4 expression in synoviocytes by high molecular weight hyaluronic acid. Biochem. Biophys. Res. Commun. 432, 580–585 (2013).
Sun, Z., Yin, Z., Liu, C. & Tian, J. The changes in the expression of NF-KB in a degenerative human intervertebral disc model. Cell. Biochem. Biophys. 72, 115–122 (2015).
Hirata, Y., Takahashi, M., Morishita, T., Noguchi, T. & Matsuzawa, A. post-translational nodifications of the TAK1-TAB complex. Int. J. Mol. Sci. 18, 205 (2017).
Demircan, K. et al. ADAMTS-9 is synergistically induced by interleukin-1beta and tumor necrosis factor alpha in OUMS-27 chondrosarcoma cells and in human chondrocytes. Arthritis Rheum. 52, 1451–1460 (2005).
Kevorkian, L. et al. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 50, 131–141 (2004).
Corps, A. N. et al. The regulation of aggrecanase ADAMTS-4 expression in human Achilles tendon and tendon-derived cells. Matrix Biol. 27, 393–401 (2008).
Moulharat, N. et al. Effects of transforming growth factor-beta on aggrecanase production and proteoglycan degradation by human chondrocytes in vitro. Osteoarthritis Cartilage 12, 296–305 (2004).
Koshy, P. J. et al. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: A time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 46, 961–967 (2002).
Kunisch, E., Kinne, R. W., Alsalameh, R. J. & Alsalameh, S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: in situ hybridization studies on a single cell level. Int. J. Rheum. Dis. 19, 557–566 (2016).
Hui, W., Rowan, A. D. & Cawston, T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-beta1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-alpha. Cytokine 16, 31–35 (2001).
Takahashi, N. et al. Elucidation of IL-1/TGF-beta interactions in mouse chondrocyte cell line by genome-wide gene expression. Osteoarthritis Cartilage 13, 426–438 (2005).
Verrecchia, F., Pessah, M., Atfi, A. & Mauviel, A. Tumor necrosis factor-alpha inhibits transforming growth factor-beta /Smad signaling in human dermal fibroblasts via AP-1 activation. J. Biol. Chem. 275, 30226–30231 (2000).
Seystahl, K., Tritschler, I., Szabo, E., Tabatabai, G. & Weller, M. Differential regulation of TGF-beta-induced, ALK-5-mediated VEGF release by SMAD2/3 versus SMAD1/5/8 signaling in glioblastoma. Neuro. Oncol. 17, 254–265 (2015).
Lu, T., Tian, L., Han, Y., Vogelbaum, M. & Stark, G. R. Dose-dependent cross-talk between the transforming growth factor-beta and interleukin-1 signaling pathways. Proc. Natl Acad. Sci. USA. 104, 4365–4370 (2007).
van der Kraan, P. M. The changing role of TGFbeta in healthy, ageing and osteoarthritic joints. Nat. Rev. Rheumatol. 13, 155–163 (2017).
Bethea, J. R., Gillespie, G. Y. & Benveniste, E. N. Interleukin-1 beta induction of TNF-alpha gene expression: involvement of protein kinase C. J. Cell Physiol. 152, 264–273 (1992).
Tan, L. et al. Studies of TAK1-centered polypharmacology with novel covalent TAK1 inhibitors. Bioorg. Med. Chem. 25, 1320–1328 (2017).
Totzke, J. et al. Takinib, a selective TAK1 inhibitor, broadens the therapeutic efficacy of TNF-alpha inhibition for cancer and autoimmune disease. Cell Chem. Biol. 24, 1029–1039 (2017). e1027.
Rodon, J. et al. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res. 21, 553–560 (2015).
Herbertz, S. et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug. Des. Devel. Ther. 9, 4479–4499 (2015).
Acknowledgements
We thank Chugai Pharmaceutical Co., LTD (Tokyo, Japan) for the gifts of HMW-HA (Suvenyl®) and tocilizumab (Actemra®). We are also grateful to Dr Kiran Chada, Professor in Rutgers-RWJMS for his kind review of this manuscript.
Funding
This study was supported by Japan Society for the Promotion of Science Grant-in-aid for Scientific Research (JSPS KAKENHI) grant (16H05454 and 19H03788 to YO). This study was also funded in part by the High Technology Research Center Grant and the Program for the Strategic Research Foundation at Private Universities (2014–2019) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Author information
Authors and Affiliations
Contributions
MZC had a role in acquisition, analysis and interpretation of the data, and drafting the article. SdV, JS, CY, and HK had a role in acquisition, analysis, and interpretation of the data. YM, MC, SM, and MI assisted in the experiments and had a role in analysis and interpretation of the data. KK had a role in conception and design. MI had a role in conception and design and drafting the article. YO had a role in conception and design and drafting the article, had a full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We obtained written informed consent from patients for experimental use of the samples. The study protocols were approved by the Keio University School of Medicine Ethics Committee and the Ethical Committee Review Board at Juntendo University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cilek, M.Z., de Vega, S., Shiozawa, J. et al. Synergistic upregulation of ADAMTS4 (aggrecanase-1) by cytokines and its suppression in knee osteoarthritic synovial fibroblasts. Lab Invest 102, 102–111 (2022). https://doi.org/10.1038/s41374-021-00685-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-021-00685-4