Abstract
Type III Bartter syndrome (BS) (OMIM607364) is caused by mutations in the basolateral chloride channel ClC-Kb gene (CLCNKB). The CLCNKB gene is sometimes reported as having a large deletion mutation, but all cases reported previously were large homozygous deletions and a large heterozygous deletion is impossible to detect by direct sequencing. This report concerns a genetic analysis of five Japanese patients with type III BS. To identify the mutations, we used polymerase chain reaction (PCR) and direct sequencing. To detect large heterozygous deletion mutations of the CLCNKB gene, we conducted semiquantitative PCR amplification using capillary electrophoresis. The result was that four mutations were identified, comprising one novel 2-bp deletion mutation, an entire heterozygous deletion, and a heterozygous deletion mutation of exons 1 and 2. The nonsense mutation W610X was detected in all patients, and this mutation is likely to constitute a founder effect in Japan. Capillary electrophoresis is a new method and extremely useful for detecting large heterozygous deletions, and should be used to examine type III BS cases in whom only a heterozygous mutation has been detected by direct sequencing. This is the first report to identify large heterozygous deletion mutations in the CLCNKB gene in patients with type III BS.
Similar content being viewed by others
Main
Bartter Syndrome (BS) is an autosomal recessive inherited disorder characterized by hypokalemic metabolic alkalosis with normal or low blood pressure despite hyperreninemia and hyperaldosteronemia. Recent genetic studies have found that this disease is caused by mutations, which lead to transporter or channel loss-of-function directly or secondary in several genes. The first gene is the SLC12A1 gene, which encodes the apical Na-K-2Cl cotransporter (NKCC2), the second is the KCNJ1 gene, which encodes the apical renal outer medullary K channel (ROMK), and the third is the CLCNKB gene, which encodes the basolateral Cl channel Kb (ClC-Kb) expressed in the thick ascending limb of Henle's loop. The mutations of these genes lead to type I–III BS (1–4). In recent years, a combination of antenatal BS and sensorineural deafness has been recognized, whose responsible gene has been identified as BSND coding barttin, a subunit for ClC-Ka and ClC-Kb, and this phenotype is now known as type IV BS (5). Moreover, the discovery of type V BS, associated with hypoparathyroidism, has been reported, and this type was found to be due to a gain of function mutations in the calcium sensing receptor, and the responsible coding gene is known as CASR (6,7).
It has previously been reported that some of the type III BS patients carry gross deletions (4,8–10), but all reported cases possess homozygous deletions, whereas heterozygous deletion mutations have not been reported because this kind of mutation is undetectable with standard PCR and sequencing. Moreover, some previously reported cases possess only heterozygous mutations and another mutations were missing (8,10). Some of these cases may attributable to the methodological problem of direct sequencing.
This report describes the results of genetic analysis of five patients with type III BS, including the detection of large heterozygous deletions in CLCNKB.
METHODS
Patients.
All procedures were reviewed and approved by the ethics committees of Kobe University.
The study was initiated after written informed consent had been obtained from all five type III BS patients and their family members. The patients were followed up at four regional hospitals in Japan: Izumiotsu Municipal Hospital, Izumiotsu; Kamitsuji Children's Clinic, Nara; Ome Municipal General Hospital, Tokyo; and Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka. BS was diagnosed based on the following four criteria: (1) severe hypokalemia (<3 mEq/L), (2) metabolic alkalosis, (3) normotensive or hypotension hyperreninemic hyperaldosteronism, (4) no drug abuse (diuretics or cathartics), anorexia nervosa, or chronic diarrhea. We could not get the consent for genetic analysis from the father of patient B.
Data collection.
Conditions of the patients' perinatal and infantile period were recorded. The lowest serum concentrations of potassium and magnesium were used for analysis.
Mutation analysis.
Total DNA was extracted and purified from peripheral leukocytes in whole-blood samples with the standard phenol-achloroform extraction method. Specific exons of CLCNKB were amplified by PCR. PCR-amplified products were purified and directly subjected to sequencing. For the sequence analysis, a Dye terminator cycle sequencing kit (Amersham Bioscience, Piscataway, NJ) was used with an automatic DNA sequencer (model ABI Prism 310; Perkin Elmer Applied Biosystems, Foster City, CA). Some of the primers for the genomic gene of CLCNKB were newly designed, as shown in Table 1, specifically to amplify the CLCNKB gene because of its >90% sequence identity with the CLCNKA gene and because primers used in previously reported studies amplified both CLCNKA and CLCNKB in some of the exons (11). To detect a large heterozygous deletion mutation in patient D and his father, we used a forward primer within the CLCNKA intron 15 whose design is as follows: CCAAGGTCTTCCGGAAGCTTG.
Semiquantitative PCR amplification.
Heterozygous gross CLCNKB gene deletion in patients C and D was detected with semiquantitative PCR amplification using capillary electrophoresis. PCR cycling conditions were as follows: initial denaturation at 94°C for 5 min followed by 20 cycles of denaturation at 94°C for 45 s, annealing at 55°–60°C for 45 s, extension at 72°C for 2 min, and a final extension at 72°C for 5 min. To quantify the amplified products, 1 μL of each reaction mixture mixed with 5 μL of the loading buffer solution containing size markers (15 and 1500 bp) was analyzed by capillary electrophoresis (Agilent 2001 Bioanalyzer with DNA 1000 Lab Chips; Agilent Technologies, Palo Alto, CA). Each of the PCR products was quantified by measuring the peak area and calculating the ratio of the key exons of the CLCNKB to exons of completely different genes for internal control. Two exon fragments, including the one corresponding to CLCNKB exons and an internal control fragment (primer pairs for SLC12A1 exon 1, 22, or SLC12A3 exon 19) were coamplified in one PCR reaction. The internal control primers were selected according to the condition of PCR amplification and PCR product size, because, to coamplify primers with CLCNKB exons in the same tube, it is necessary to have exactly the same PCR conditions to get equally amplified PCR products and the product must have a different size for electrophoresis. All family members of patients C and D and normal control subjects were analyzed at the same time with this method.
RESULTS
Patient characteristics.
Table 2 shows the patients' clinical features. Patients A-1, A-2, and C showed antenatal clinical symptoms, but none of the five patients had nephrocalcinosis. From the laboratory data, renal function was within normal range at this time.
Table 3 shows the patients' laboratory data. All of them had low serum potassium and high plasma renin activity and, except for patient C, a relatively low serum magnesium level.
Mutational analysis and semiquantitative PCR amplification.
Table 4 shows the mutations in the CLCNKB gene of all patients.
Patients A-1 and B-2.
A homozygous mutation of CLCNKB, W610× (c.1830G>A in exon 16), was detected. Both of their parents were found to carry the heterozygous W610X mutation, but their older brother was not. There were no apparent clinical symptoms or laboratory data abnormalities (Fig. 1A).
Detection of CLCNKB mutations by direct sequencing. (A) Homozygous nonsense mutation of CLCNKB, W610X (c.1830G>A in exon 16) in patients A-1 and A-2. (B) Heterozygous nonsense mutation of CLCNKB, W610X (c.1830G>A in exon 16: top) and novel heterozygous deletion mutation of CLCNKB, c.1334_1335delCT, in exon 13 (bottom) in patient B. (C) Homozygous nonsense mutation of CLCNKB, W610X (c.1830G>A in exon 16) in patient C. Although the patient's mother showed heterozygous nonsense mutation of W610X, her father did not. (D) a) Heterozygous nonsense mutation of CLCNKB, W610X (c.1830G>A in exon 16) in patient D. The patient's mother showed heterozygous nonsense mutation of W610X. b) Heterozygous large deletion mutation from CLCNKA exon 16 to CLCNKB intron 2 was detected in patient D using primer pairs whose position is shown in Figure 4. The 5′ breakpoint was the 46 base pair of the CLCNKA exon 16 and the 3′ was within the intron 2 (exon 3–185 base pair) of the CLCNKB.
Patient B.
A compound heterozygous mutation of CLCNKB, W610X (c.1830G>A in exon 16) and a frame shift at codon 445 (c.1334_1335delCT in exon 13) were detected. The small deletion mutation of c.1334_1335delCT constitutes a novel mutation. The patient's mother was found to carry the heterozygous W610X mutation, whereas neither of his parents showed any apparent clinical symptoms or laboratory data abnormalities (Fig. 1B).
Patient C.
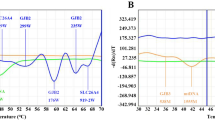
The results of direct sequencing suggested a homozygous mutation of CLCNKB, W610X (c.1830G>A in exon16), and the patient's mother carried the heterozygous W610X mutation, but the patient's father did not (Fig. 1C). Moreover, the single nucleotide polymorphisms (SNPs) analysis of c.1971T>C (in exon 18) showed that the father carried the homozygote of T but the patient the homozygote of C (Fig. 2C). The father and the patient were therefore expected to possess a large heterozygous deletion of the CLCNKB gene and the result of the direct sequencing appears to indicate that the patient carried a homozygous W610 mutation. That is to say, the patient was believed to possess a compound heterozygous mutation of the nonsense mutation and a large deletion of CLCNKB including exons 16 and 18. Following these findings, we conducted semiquantitative PCR amplification using capillary electrophoresis. The results disclosed that the patient and her father had half the quantity of PCR products of all CLCNKB exons as did her mother and the normal control (only the results of exons 1 and 19 are shown in Fig. 3A, B), indicating a reduction in the copy numbers in the entire CLCNKB gene of the patient and her father. This means that the patient had a compound heterozygous mutation of W610X and deletion of the entire CLCNKB. Her parents had no apparent clinical symptoms or laboratory data abnormalities.
Haplotype reconstruction relative to five markers (four SNPs and one mutation) in the CLCNKB of four families. Markers from the top: c.80G>T (in exon 1), c.1741C>T (in exon 15), c.1830G>A (W610X), IVS16 + 68T>C (in intron 16), and c.1971T>C (in exon 18). *We could not get the consent for genetic analysis from the father of the patient B.
Semiquantitative PCR amplification of the CLCNKB gene. Capillary electrophoretic patterns of PCR products. (A, B) Patient C. (A) CLCNKB exon 1 and SLC12A1 exon 1 (internal control). (B) CLCNKB exon 19 and SLC12A1 exon 22 (internal control). The peak areas of CLCNKB exons 1 and 19 in the patient and her father are only about half of those in her mother and the normal control. (C, D) Patient D. (C) CLCNKB exon 2 and SLC12A1 exon 1 (internal control). (D) CLCNKB exon 3 and SLC12A3 exon 19 (internal control). In the patient and her father, the peak areas of CLCNKB exon 2 is only about half and those of exon 3 is the same as in her mother and the normal control.
Patient D.
Only a heterozygous mutation of CLCNKB, W610X (c.1830G>A in exon 16), was detected and the patient's mother was found to carry the heterozygous W610X mutation (Fig. 1D–a). However, the SNPs analysis of c.80G>T (in exon 1) showed that the father carried the homozygote of T and the patient the homozygote of G (Fig. 2D). These results suggested that the patient and her father might have a large deletion around exon1. We conducted semiquantitative PCR amplification using capillary electrophoresis. The results showed that the patient and her father had half the quantity of PCR products of CLCNKB exons 1 and 2 than her mother and the normal control and the same quantity of exon 3 to exon 19 (only the results of exons2 and 3 are shown in Figure 3C, D). These results indicated a reduction in the copy numbers in exons 1 and 2 of CLCNKB gene of the patient and her father. This means that the patient had a compound heterozygous mutation of W610X and CLCNKB exon 1 and 2 deletion. Following these results, we conducted an analysis to detect the breakpoint of the large heterozygous deletion mutation by a genome-walking technique toward the CLCNKA gene and revealed that the 5′ deletion breakpoint was located in exon 16 of the CLCNKA gene. The forward primer within intron 15 of the CLCNKA gene and the reverse primer within intron 3 of the CLCNKB gene were used for PCR and generated a 522-bp amplicon in the patient and the father, who was suggested to have a heterozygous large deletion mutation by semiquantitative PCR (Fig. 4). The result of the direct sequencing of this PCR product shows the breakpoint of the large deletion (Fig. 1D-b). Her parents showed no apparent clinical symptoms or laboratory data abnormalities.
Detection of large heterozygous deletion mutation in patient D. The forward primer within intron 15 of the CLCNKA gene and the reverse primer within intron 3 of the CLCNKB gene are indicated by arrows. Amplification by PCR with this primer pair generated a 522-bp amplicon, which was detected in patient and the father, who was suggested to have a heterozygous large deletion mutation by semiquantitative PCR. The result of the direct sequencing of this PCR product is shown in Figure 1D-b).
Generation of polymorphic markers to the CLCNKB gene.
All patients and family members were examined for markers of four SNPs and c.1830G>A (W610X) mutation. The findings for the four SNPs were as follows: c.80G>T (in exon 1), c.1741C>T (in exon 15), c.1845 + 68T>C (IVS16 + 68), and c.1971T>C (in exon 18). The results of haplotype construction for the four family members are shown in Figure 2. The loci with c.1830A (W610X) mutation had exactly the same polymorphic pattern, suggesting that the W610X mutation may constitute a founder effect in the Japanese population.
SNPs in CLCNKB.
A number of nucleotide exchanges were detected in this study. Some were silent mutations without an amino acid exchange. Others led to an amino acid exchange, but were detected also in healthy control subjects or reported previously as polymorphisms. Those were implicated the following amino acids: F4L (c.10T>C), R27L (c.80G>T), S108S (c.324A>G), S195I (c.584G>T), Q197 K (c.589C>A), A287V (c.860C>T), Q356Q (c.1068G>A), S195I (c.584G>T), M562T (c.1685T>G), K578E (c.1732A>G), L581L (c.1741C>T), F657F (c.1971T>C), P683P (c.2049G>T), IVS5-5c>t, IVS10 + 5g>a, IVS16 + 68T>C.
DISCUSSION
In the study presented here, several mutations were identified, including one novel mutation, and heterozygous gross deletion mutations in five Japanese patients with type III BS, all of whom met the clinical criteria for antenatal or classic BS. Genetic diagnosis confirmed that all the patients had type III BS.
Type III BS is generally classified as classic BS because patients are usually diagnosed while they are infants and lack nephrocalcinosis or renal insufficiency; characteristics that differentiate patients with type III BS from type I and II BS (2,3,8,12,13). BS patients show hypercalciuria and normal magnesium levels. Recently, however, some studies have disclosed that the clinical character of type III BS involves a highly variable phenotype ranging from episodes of severe dehydration during the neonatal period to almost asymptomatic patients diagnosed during adolescence. Moreover, some cases feature hypocalciuria and/or hypomagnesemia, which sometimes mislead the type III BS to Gitelman syndrome, as reported previously (8,12,14).
The results of our study raise two novel points in genetic analysis of type III BS cases.
The first point concerns the W610X nonsense mutation in CLCNKB. This mutation was previously detected in six unrelated Japanese BS patients (11,14,15) and was found in all five patients in our study. Most of these cases are from completely disparate localities in Japan, which leads to the hypothesis that this may be a founder mutation in Japan. In fact, haplotype construction using polymorphic markers localized at the CLCNKB locus for these families suggests a common ancestry.
The second point concerns the detection of large heterozygous deletion mutations. These kinds of mutations are very difficult to detect because they are undetectable with a standard PCR and direct sequencing. The most commonly applied techniques to detect these mutations are fluorescent in situ hybridization (FISH) and Southern blotting. FISH analysis has been preferred because it allows for visual determination of the copy number in the region examined. However, the method is complicated, expensive, and time-consuming. Southern blot is simpler than FISH, but depends greatly on technical skill and is more laborious than semiquantitative PCR. To date, some authors have used capillary electrophoresis to detect heterozygous deletions (16,17), as did we in our study, and clearly detected a large heterozygous deletion. Previously, several authors have reported the presence of gross homozygous deletions in the CLCNKB gene, and it is relatively easy to find a wide range of deletions in this gene (4,8–10). This mechanism has recently been clarified and named the rearrangement theory (18). The clarification was facilitated by the presence of the region specific low-copy repeats CLCNKA and CLCNKB, which have more than 90% sequence identity. Some previous reports have dealt with BS cases with only a heterozygous mutation in CLCNKB (4,7,8,15), but the missing mutations in some of these cases may be attributable to the methodological problem of direct sequencing. Ours is thus the first report to identify large heterozygous deletion mutations in the CLCNKB gene in patients with type III BS. Moreover, we can also say that in cases with one homozygous mutation, there remains the possibility that this homozygosity is due to the complete absence of the second allele, as seen in patient C in this report. This can only be clarified by family analysis. But in routine genetic testing, it is conducted mainly in single sporadic patients using direct sequencing, and in this situation, large heterozygous deletions may sometimes be missing.
To summarize, we used direct sequencing and semiquantitative PCR amplification to identify one novel mutation and two large heterozygous CLCNKB deletions in five Japanese patients with type III BS, and ours is the first study using this method to detect a large heterozygous deletion in inherited kidney disease. This kind of mutation warrants investigation in patients with type III BS with only a heterozygous mutation. Furthermore, we hypothesize that the nonsense mutation W610X is a founder effect of Japanese population.
In conclusion, type III BS cases with only a heterozygous mutation detected by means of direct sequencing should be further examined for detection of the possible presence of a large heterozygous deletion mutation.
Abbreviations
- BS:
-
Bartter syndrome
- ClC-Kb:
-
Cl channel KbNKCC2 Na-K-2Cl cotransporter
- ROMK:
-
renal outer medullary K channel
- SNPs:
-
single nucleotide polymorphisms
References
Bartter FC, Pronove P, Gill JR Jr, Maccardle RC 1962 Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med 33: 811–828
Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP 1996 Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188
Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP 1996 Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156
Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP 1997 Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 17: 171–178
Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F 2001 Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29: 310–314
Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaitre X, Paillard M, Planelles G, Dechaux M, Miller RT, Antignac C 2002 Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13: 2259–2266
Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T 2002 Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet 360: 692–694
Konrad M, Vollmer M, Lemmink HH, van den Heuvel LP, Jeck N, Vargas-Poussou R, Lakings A, Ruf R, Deschenes G, Antignac C, Guay-Woodford L, Knoers NV, Seyberth HW, Feldmann D, Hildebrandt F 2000 Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol 11: 1449–1459
Colussi G, De Ferrari ME, Tedeschi S, Prandoni S, Syren ML, Civati G 2002 Bartter syndrome type 3: an unusual cause of nephrolithiasis. Nephrol Dial Transplant 17: 521–523
Tajima T, Nawate M, Takahashi Y, Mizoguchi Y, Sugihara S, Yoshimoto M, Murakami M, Adachi M, Tachibana K, Mochizuki H, Fujieda K 2006 Molecular analysis of the CLCNKB gene in Japanese patients with classic Bartter syndrome. Endocr J 53: 647–652
Fukuyama S, Okudaira S, Yamazato S, Yamazato M, Ohta T 2003 Analysis of renal tubular electrolyte transporter genes in seven patients with hypokalemic metabolic alkalosis. Kidney Int 64: 808–816
Jeck N, Konrad M, Peters M, Weber S, Bonzel KE, Seyberth HW 2000 Mutations in the chloride channel gene, CLCNKB, leading to a mixed Bartter-Gitelman phenotype. Pediatr Res 48: 754–758
Peters M, Jeck N, Reinalter S, Leonhardt A, Tonshoff B, Klaus GG, Konrad M, Seyberth HW 2002 Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med 112: 183–190
Fukuyama S, Hiramatsu M, Akagi M, Higa M, Ohta T 2004 Novel mutations of the chloride channel Kb gene in two Japanese patients clinically diagnosed as Bartter syndrome with hypocalciuria. J Clin Endocrinol Metab 89: 5847–5850
Watanabe T, Tajima T 2005 Renal cysts and nephrocalcinosis in a patient with Bartter syndrome type III. Pediatr Nephrol 20: 676–678
White SJ, Sterrenburg E, van Ommen GJ, den Dunnen JT, Breuning MH 2003 An alternative to FISH: detecting deletion and duplication carriers within 24 hours. J Med Genet 40: e113
Thomas AC, Cullup T, Norgett EE, Hill T, Barton S, Dale BA, Sprecher E, Sheridan E, Taylor AE, Wilroy RS, Delozier C, Burrows N, Goodyear H, Fleckman P, Stephens KG, Mehta L, Watson RM, Graham R, Wolf R, Slavotinek A, Martin M, Bourn D, Mein CA, O'Toole EA, Kelsell DP 2006 ABCA12 is the major harlequin ichthyosis gene. J Invest Dermatol 126: 2408–2413
Stankiewicz P, Lupski JR 2002 Genome architecture, rearrangements and genomic disorders. Trends Genet 18: 74–82
Acknowledgements
The authors thank Yoshimi Nozu for her help in genetic analysis.
Author information
Authors and Affiliations
Additional information
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan Running.
Rights and permissions
About this article
Cite this article
Nozu, K., Fu, X., Nakanishi, K. et al. Molecular Analysis of Patients With Type III Bartter Syndrome: Picking Up Large Heterozygous Deletions With Semiquantitative PCR. Pediatr Res 62, 364–369 (2007). https://doi.org/10.1203/PDR.0b013e318123fb90
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318123fb90
This article is cited by
-
Detection of copy number variations by pair analysis using next-generation sequencing data in inherited kidney diseases
Clinical and Experimental Nephrology (2018)
-
Mixed Bartter-Gitelman syndrome: an inbred family with a heterogeneous phenotype expression of a novel variant in the CLCNKB gene
SpringerPlus (2014)
-
Natural history of genetically proven autosomal recessive Alport syndrome
Pediatric Nephrology (2014)
-
Bartter syndrome in two sisters with a novel mutation of the CLCNKB gene, one with deafness
European Journal of Pediatrics (2011)
-
Hypokalemic rhabdomyolysis in a child with Gitelman’s syndrome
Pediatric Nephrology (2010)