Abstract
Deafness is a common sensory disorder. In China, approximately 70% of hereditary deafness originates from four common deafness-causing genes: GJB2, SLC26A4, GJB3, and MT-RNR1. A single-tube rapid detection method based on 2D-PCR technology was established for nine mutation sites in the aforementioned genes, and Sanger sequencing was used to verify its reliability and accuracy. The frequency of hotspot mutations in deafness genes was analysed in 116 deaf students. 2D-PCR identified 27 genotypes of nine loci according to the melting curve of the FAM, HEX, and Alexa568 fluorescence channels. Of the 116 deaf patients, 12.9% (15/116) carried SLC26A4 mutations, including c.919-2A > G and c.2168A > G (allele frequencies, 7.3% and 2.2%, respectively). The positivity rate (29.3%; 34/116) was highest for GJB2 (allele frequency, 15.9% for c.235delC, 6.0% for c.299_300delAT, and 2.6% for c.176-191del16). Sanger sequencing confirmed the consistency of results between the detection methods based on 2D-PCR and DNA sequencing. Common pathogenic mutations in patients with non-syndromic deafness in Changzhou were concentrated in GJB2 (c.235delC, c.299_300delAT, and c.176-191del16) and SLC26A4 (c.919-2A > G and c.2168 A > G). 2D-PCR is an effective method for accurately and rapidly identifying deafness-related genotypes using a single-tube reaction, and is superior to DNA sequencing, which has a high cost and long cycle.
Similar content being viewed by others
Introduction
Currently, > 1.5 billion people worldwide suffer from hearing loss, including 34 million children, 60% of whom have preventable causes1. Congenital hearing loss is a great concern in the medical field. It is one of the most common diseases and is diagnosed in 1–2 newborns per 1,000 live births2. Among prepubertal individuals, the incidence increases to 3.5 per 1,000 individuals3. This disease is highly heterogeneous. Each person has unique etiological characteristics of hearing loss depending on ethnic, geographic, social, and medical factors. In industrialised countries, genetic factors account for two-thirds of the cases of congenital hearing loss4. Most affected individuals (approximately 70%) have non-syndromic hearing loss (NSHL)5, which is characterised by no disease other than hearing loss. Autosomal inheritance is the most common mode of inheritance in patients with NSHL6. In the Chinese population, it has been demonstrated that NSHL is closely associated with genetic polymorphisms of gap junction protein β2 (GJB2), gap junction protein β3 (GJB3), solute carrier family 26 member 4 (SLC26A4), and mitochondrial DNA (mtDNA) 12S ribosomal RNA (12S rRNA)7. Currently, traditional physical screening for hearing defects is widely used, and is associated with the risk of missing detection. The detection of susceptibility genes for deafness can facilitate early detection, intervention, and treatment to clarify the cause, avoid the induction of deafness, and guide the use of drugs. Therefore, detection of deafness susceptibility genes is of great significance. At present, the common screening methods for deafness susceptibility genes are the melting curve8, gene chip (including the microarray method)9, Sanger sequencing10, and second-generation sequencing methods (including whole-exome sequencing, WES)11.
Two-dimensional PCR (2D-PCR) is based on the base-quenching probe technology previously developed by our group and is mainly used for the detection of single-nucleotide polymorphisms12,13,14,15,16,17. Presently, our team has successfully established a single-tube method for the differential diagnosis of nine types of high-risk human papillomaviruses using 2D-PCR18. In the present study, 2D-PCR was used to detect the GJB2, SLC26A4, GJB3, and MT-RNR1 genes in 116 deaf patients and to explore the diagnosis rate and mutation patterns of four common pathogenic genes for deafness in Changzhou, Jiangsu, China.
Materials and methods
Patients and DNA samples
Whole-blood samples (2.5 ml) from 116 unrelated deaf students in Changzhou Deaf School were collected in June 2010, and stored at -80 ℃ (Changzhou, Jiangsu, China), including samples from 61 male and 55 female patients with an average age of 14.6 ± 4.03 (mean ± SD) years. Each participant provided written informed consent, and informed consent was obtained from the guardians of minors. This study was approved by the Ethics Committee of Changzhou First People’s Hospital (Changzhou, China). All methods were performed in accordance with the relevant guidelines and regulations. Genomic DNA was extracted from peripheral blood samples using a TIANamp Blood DNA kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions.
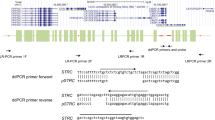
Primers, probes and reaction conditions
The primer design combined the design principles of 2D-PCR18 and Amplification Refractory Mutation System PCR (ARMS-PCR). The last base of the 3ʹ end of the primer, with a tag, specifically recognised the wild-type and mutant templates. To improve the amplification specificity, an artificial mismatch base was introduced at the first 3–6 bases of the 3ʹ end of the primer with tag. All primers and probes were designed using Primer Premier 5.0 software. According to the principles of 2D-PCR, three different sequences were designed as probes and labelled with carboxyfluorescein (FAM), hexachloro-fluorescein (HEX), and Alexa568, respectively, and the corresponding three sets of sequences homologous to the corresponding probe sequence were designed as tags. Forward primers were distributed in the three fluorescence channels after being connected to the tags. All primers, tags, and probe sequences are listed in Table 1. Sanger sequencing of all samples and the synthesis of primers and probes were completed by Sangon Biotech Co., Ltd., Shanghai, China; sequencing primers are shown in Table 2. The 2D-PCR reaction mixture contained 2.5 μl 10 × ImmoBuffer (Bioline; Meridian Bioscience, Inc., Cincinnati, OH, USA), 0.75 μl 50 mM MgCl2 (Bioline; Meridian Bioscience, Inc.), 0.5 μl 5 U/μl IMMOLASE DNA polymerase (Bioline; Meridian Bioscience, Inc.), 0.7 μl 2.5 mM deoxynucleotide triphosphates (Takara Bio, Inc., Otsu, Japan), 1 μl of 10 μM probe (FAM), 0.8 μl of 10 μM probe (HEX and Alexa568), 0.05–0.2 μl of each 10 μM primer with tag, 0.8–1 μl of the 10 μM primer (see Supplementary Table 1 for details), deionised water to a total volume of 23 μl and, finally, 2 μl of the sample. A reaction mix system was prepared before amplification, and all reagents were stored independently. Because each probe was labelled with a fluorophore, it was stored away from light. Taq enzymes must also be stored independently. The PCR programs for the hot-start reaction system were as follows: pre-incubation at 95˚C for 10 min; 5 cycles at 95˚C for 10 s and 60˚C for 10 s; 35 cycles at 95˚C for 10 s, 72˚C for 1 s and 60˚C for 10 s. The fluorescence acquisition began with heating at 95˚C for 30 s and 30˚C for 4 min; the temperature was gradually increased from 30 to 70˚C with a ramp rate of 0.06˚C/sec, and the fluorescence signal was acquired continuously. Fluorescence intensity was measured using three detection channels: FAM, HEX, and Alexa568. Amplification and melting curve analyses were performed using a SLAN-96S real-time PCR machine (Hongshi Tech, Shanghai, China).
Statistical analysis
Results are expressed as mean ± the SD. Chi-square test was used to compare the detection rates of hereditary deafness gene mutations between Changzhou and other areas in China. GraphPad Prism 8.0 software (GraphPad Software; DotMatics, Boston, MA, USA) was used to perform statistical analyses. P < 0.05 was considered statistically significant.
Ethics approval
Each participant provided written informed consent, and informed consent was obtained from their guardians. This study was approved by the Ethics Committee of Changzhou First People’s Hospital (Changzhou, China).
Results
2D-PCR melting temperatures
The genotypes of nine loci in 116 deaf patients were detected using 2D-PCR and Sanger sequencing. Wild-type or homozygous mutant plasmid (5 μl each; 1 × 106 copies) from the nine sites were mixed to a final concentration of approximately 1 × 105 copies, and the mixed plasmids were used as templates for 2D-PCR amplification. The melting curves are shown in Fig. 1A,B. The melting temperatures of different genotypes at each locus detected by 2D-PCR are listed in Table 3. According to the melting curve and the corresponding melting temperature, the melting curves of FAM channels were all wild-type; those of the HEX channel at < 48˚C were wild-type and that at > 48˚C was mutant type; and the melting curves of the Alexa568 channel were all mutant types.
2D-PCR plasmid amplification melting curves. (A) Melting curve of wild-type mixed plasmid (1 × 105 copies). (B) Melting curve of mutant mixed plasmid (1 × 105 copies). 919–2, c.919-2A > G; 235, c.235delC; 176, c.176-191del16; 299, c.299_300delAT; 1229, c.1229C > T; 2168, c.2168A > G; 538, c.538C > T; 1494, m.1494C > T; 1555, m.1555A > G; 2D-PCR, two-dimensional PCR; M, mutant type; W, wild-type. Blue melting curve represents the FAM channel; green melting curve represents the HEX channel; orange melting curve represents the Alexa568 channel.
Frequency of nine hotspot mutations
A total of 50 of 116 patients (43.1%) carried at least one genetic deafness-associated variant (one patient had mutations in both GJB3 and MT-RNR1). Table 4 shows the genotypes of 116 deaf patients from the Changzhou area. Table 5 shows the allele frequencies of the nine hotspot mutations associated with deafness.
GJB2
A total of 34 patients with GJB2 gene mutations were detected by the 2D-PCR and Sanger sequencing, including 13 homozygous and 21 heterozygous mutations. There were 10 homozygous (the results of 2D-PCR and sequencing are shown in Fig. 2A) and 17 heterozygous (Fig. 2B) c.235delC mutations (positivity rate, 23.3%; frequency of mutant alleles, 15.9%), 2 homozygous (Fig. 2C) and 10 heterozygous (Fig. 2D) mutations of c.299_300delAT (positivity rate, 10.3%; frequency of mutant alleles, 6.0%), and 1 homozygous (Fig. 2E) and 4 heterozygous mutations (all double-site heterozygous mutations) of c.176-191del16 (positivity rate, 4.3%; frequency of mutant alleles, 2.6%). A patient with c.35insG was identified using Sanger sequencing; this patient also carried the c.299_300delAT mutation (see Supplementary Fig. 1 for details). In addition, 10 patients were compound heterozygous, accounting for 29.4% (10/34) of the total GJB2 gene mutations. Of these, 17.6% (6/34) carried c.235delC + c.299_300delAT (Fig. 3A), 8.8% (3/34) carried c.235delC + c.176-191del16 (Fig. 3B), and 2.9% (1/34) carried c.299_300delAT + c.176-191del16 (Fig. 3C).
Melting curve of GJB2 gene single point mutation and its corresponding Sanger sequencing map. (A) c.235delC/c.235delC homozygous mutation. (B) c.235delC/wt heterozygous mutation. (C) c.299_300delAT/c.299_300delAT homozygous mutation. (D) c.299_300delAT/wt heterozygous mutation. (E) c.176-191del16/c.176-191del16 homozygous mutation. The mutation site is underlined and the red arrow points to the mutation position. 235, c.235delC; 176, c.176-191del16; 299, c.299_300delAT; GJB2, gap junction protein β2; M, mutant type; W, wild-type.
Melting curves and corresponding Sanger sequencing results for the GJB2 compound heterozygous mutation. (A) c.235delC/c.299_300delAT. (B) c.235delC/c.176-191del16. (C) c.176-191del16/c.299_300delAT. The mutation site is underlined and the red arrow points to the mutation position. 235, c.235delC; 176, c.176-191del16; 299, c.299_300delAT; GJB2, gap junction protein β2; M, mutant type; W, wild-type.
The positivity rate and allele frequency of c.235delC were the highest (23.3% and 15.9%, respectively), similar to the results of studies performed in Jiangsu (20.6%; χ2, 1.58; P > 0.05) and Shanghai (17.7%; χ2, 0.12; P > 0.05)19. However, the positivity rate was significantly higher than that reported for 3,004 patients from 26 regions in China (16.3%; χ2, 4.01; P < 0.05), but there was no significant difference in allele frequencies (15.9 vs. 12.0%; χ2, 3.26; P > 0.05)20.
In the present study, the positivity rates of c.299_300delAT and c.176-191del16 in Changzhou were 10.3 and 4.3%, respectively, and the allele frequencies were 6.0 and 2.6%, respectively, which were not significantly different from those reported in Jiangsu and Shanghai. However, the positivity rates and allele frequencies of c.299_300delAT and c.176-191del16 in this study were significantly higher than those of 2,063 patients from 23 provinces in China (c.299_300delAT: positivity rate, 4.4%; χ2, 8.81; P < 0.01; allele frequency, 2.4%; χ2, 11.75; P < 0.001; c.176-191del16: positivity rate, 1.4%; χ2, 6.03; P < 0.05; allele frequency, 0.75%; χ2, 8.79; P < 0.01)19. Figure 4 shows the gene frequencies of three hotspot mutations in the GJB2 gene in the present cohort from Changzhou and its surrounding areas.
Distribution of allele frequencies of three hotspot mutations (c.235delC, c.299_300delAT and c.176-191del16) of the GJB2 gene in the study cohort from Changzhou and its surrounding provinces and cities, as well as in China (15,16). The participants in all regions were deaf patients. GJB2, gap junction protein β2.
SLC26A4
SLC26A4 mutations were detected in 15 of the 116 patients. There were 3 homozygous (Fig. 5A) and 11 heterozygous (Fig. 5B) c.919-2A > G mutations (positivity rate, 12.1%; frequency of mutant alleles, 7.3%), and 5 heterozygous (Fig. 5C) c.2168A > G mutations (positivity rate, 4.3%; frequency of mutant alleles, 2.2%). No mutation at position c.1229C > T was detected. Four patients (26.7%; 4/15) exhibited mutations at multiple sites in SLC26A4. Of these patients, three carried the c.919-2A > G + c.2168A > G mutation (Fig. 5D). Another patient had three mutations simultaneously (SLC26A4 c.919-2A > G + c.2168A > G + MT-RNR1 gene m.1555A > G; Fig. 6A).
Melting curves of SLC26A4 genotypes and their corresponding Sanger sequencing results. (A) c.919-2A > G/c.IVS7-2A > G homozygous mutation. (B) c.919-2A > G/wt heterozygous mutation. (C) c.2168A > G/wt heterozygous mutation. (D) c.919-2A > G/c.2168A > G compound heterozygous mutation. The red arrow points to the mutation site. 919–2, c.919-2A > G; 2168, c.2168A > G; M, mutant type; SLC26A4, solute carrier family 26 member 4; W, wild-type.
Melting curves and sequencing maps of patients with three mutation sites and the m.1555A > G homogenous variant alone. (A) SLC26A4 c.919-2A > G + c.2168A > G + MT-RNR1 gene m.1555A > G. (B) m.1555A > G homoplasmic. The red arrow points to the mutation site. 919–2, c.919-2A > G; 2168, c.2168A > G; 1555, m.1555A > G; M, mutant type; mtDNA, mitochondrial DNA; SLC26A4, solute carrier family 26 member 4; W, wild-type.
The present study revealed the highest allele mutation rate of c.919-2A > G (7.3%) in the SLC26A4 gene, which was significantly higher than that reported for cohorts from other cities in East China, such as Wenzhou21 (3.66%; χ2, 24.64; P < 0.0001) and Xiamen22 (6.13%; χ2, 5.19; P < 0.05). In 2,352 patients with non-syndromic deafness from 27 cities in China, the positivity rates of three known pathogenic sites of the SLC26A4 gene, c.919-2A > G, c.2168A > G, and c.1229C > T, were 11.52, 2.5%, and 0.51%, respectively. The allele frequencies were 8.01, 1.51, and 0.25%, respectively23. Figure 7 shows the gene frequencies of three hotspot mutations in the SLC26A4 gene in the present cohort from Changzhou and its surrounding areas. The positivity rate and allele frequency of the two major SLC26A4 mutation sites, c.919-2A > G and c.2168A > G, in the present cohort from the Changzhou area were similar to those at the national level, and there was no significant difference.
Distribution of allele frequencies of two hotspot mutations (c.919-2A > G and c.2168A > G) of the SLC26A4 gene in the study cohort from Changzhou and its surrounding provinces and cities, as well as the whole country (15–17). The participants in all regions were deaf patients. SLC26A4, solute carrier family 26 member 4.
MT-RNR1 and GJB3
A total of 2 cases of MT-RNR1 gene mutation were detected (both m.1555A > G homoplasmic mutation; Fig. 6B). The positivity and allelic mutation rates were 1.7% and 1.7%, respectively. The mutation at c.538C > T of the GJB3 gene was not detected in any patients.
Comparison of 2D-PCR and Sanger sequencing results
The 2D-PCR method for the 35delG locus of GJB2 was not included in this study. Therefore, the 2D-PCR results for the nine loci were consistent with the Sanger sequencing results. The kappa test showed complete concordance between 2D-PCR and the Sanger sequencing methods (k = 1; P = 0.000).
Discussion
Deafness is a common sensory disorder with a complex aetiology; approximately 60% of deafness is hereditary24. According to the Report on Prevention and Treatment of Birth Defects in China in 201225, hearing disabilities accounted for 24.97% of the disabled population in China. With the improvement of healthcare consciousness of eugenics, the causes of deafness caused by environmental factors have been gradually reduced, and the proportion of hereditary deafness is gradually increasing; thus, the detection of deafness gene mutations is increasingly important.
The present study demonstrates that the GJB2 gene had the highest mutation detection rate. Connexin 26 (Cx26), encoded by GJB2, is a gap junction protein composed of six monomers26. In fact, deleterious mutations in the gene GJB2 encoding Cx26 underlie the most common form of non-syndromic congenital deafness, making it an important putative target for gene therapy27,28. Research demonstrated that GJB2 gene mutations can cause potassium repolarization reflux disorders, resulting in hearing loss29. If newborn deafness gene detection reveals that the infant exhibits a double mutation in the GJB2 gene at any site of c.35delG, c.176-191del16, c.235delC, or c.299_300delAT, the auditory nerve, auditory conduction pathway, and speech centre of the infant are normal, and a good rehabilitation effect can be obtained by cochlear implantation30. GJB2 is the most common deafness-causing gene in many ethnic groups. Among GJB2 mutations, c. 235delC is the most common in Asian populations31,32,33,34. The positivity rates of GJB2 c.235delC, c.299_300delAT, and c.176-191del16 have been reported to be 16.320, 4.36, and 1.4%, respectively19. The positivity rates of GJB2 c.235delC, c.299_300delAT, and c.176-191del16 were 23.3, 10.3, and 4.3%, respectively, in patients with non-syndromic hearing loss in the present study cohort from Changzhou. In addition, nine patients had c.235delC with other mutations, accounting for 52.9% (9/17) of the total heterozygous mutations, including six cases of c.235delC + c.299_300delAT and three cases of c.235delC + c.176-191del16. The heterozygous mutation c.235delC is often accompanied by another mutation that causes deafness. Therefore, the GJB2 c.235delC, c.299_300delAT, and c.176-191del16 loci can be used as the focus of genetic screening for deafness in Changzhou to avoid blind screening.
In addition, Sanger sequencing in the present study revealed a novel compound heterozygous mutation, c.35insG + c.299_300delAT, in a study cohort from the Changzhou area. It has been reported that 35insG insertions lead to frameshift mutations in sporadic deafness cases35,36,37. However, to the best of our knowledge, no compound heterozygous c.35insG + c.299_300delAT mutations have been reported. c.35insG caused the stop codon to advance to position 47, and the translated amino acid was shortened accordingly, resulting in a truncated protein that could not function normally. Because the 3ʹ end of 35delG site has GGGGGG, which increases the difficulty of specific primer design, we will optimise the specific primer sequence of this site. In future experiments, 2D-PCR detection of c.35delG and c.35insG will be performed.
Notably, none of the patients included in the present study had a history of cancer. Previous studies have found that GJB2 is highly expressed in lung adenocarcinoma38, cervical cancer39, breast cancer40, and pancreatic cancer41, and is associated with poor prognosis. However, GJB2-related deafness susceptibility genes are not associated with cancer. This may be related to the increased expression of GJB2 and the degree of differentiation of different cancers, indicating that GJB2 can be used as a potential prognostic marker and therapeutic target for the poor survival of cancer patients. However, in deaf patients, GJB2 mutations may cause abnormal expression of GJB2 mRNA or protein, resulting in the loss of GJB2 expression11. Therefore, GJB2 mutations associated with deafness may play a role in tumour suppression to some extent.
Mutation of the SLC26A4 gene is responsible for enlargement of the vestibular aqueduct42. In China, approximately 97% of enlarged vestibular aqueducts are caused by SLC26A4 gene mutations43. Among the SLC26A4 gene mutations, c.919–2 A > G has the highest mutation rate in the Chinese population. In 2,352 patients with non-syndromic deafness from 27 cities in China, the positivity rates of c.919–2 A > G, c.2168 A > G, and c.1229 C > T were 11.5, 2.5, and 0.5%, respectively23. In the present study, the positivity rates of these three loci were 7.3, 2.2, and 0%, respectively. Compared with the aforementioned reports, the mutation trend at each site was similar; however, no mutation was found at the c.1229 C > T site in the study cohort from the Changzhou area, which may be related to different genetic backgrounds. Furthermore, four of the five patients (80%) with heterozygous c.2168 A > G mutations also had a heterozygous c.919–2 A > G mutation. Therefore, the c.919–2 A > G and c.2168 A > G loci are hotspot mutations in patients with SLC26A4 gene mutations in the Changzhou area, which together affect the hearing of patients with large vestibular aqueducts.
mtDNA is the cytoplasmic genome that is independent of nuclear chromosomes. MT-RNR1 m.1555A > G and m.1494C > T mutations are closely associated with aminoglycoside-induced deafness44. Mutant carriers are extremely sensitive to aminoglycosides and tinnitus and severe deafness may occur even with low doses of aminoglycosides. Epidemiological data demonstrate that mtDNA m.1555A > G and m.1494C > T are the two main pathogenic loci of the MT-RNR1 gene in the Chinese deaf population, with carrying rates of 3.4345 and 0.41%46. In the present study, the positivity rates of m.1555A > G and m.1494C > T were 1.7% and 0%, respectively; the difference from our previous data was due to the loss of a sample from a patient with a homoplasmic A-to-G mutation at position 1555 of the mtDNA in the present study, with the total number of participants changing from 117 to 116. Although this result was lower than the national level, the difference was not statistically significant. This was due to the different numbers of participants and different genetic backgrounds caused by regional and ethnic differences. In the Changzhou non-syndromic deafness population, the m.1555A > G mutation is common in patients with drug-induced deafness. In addition, among the four patients with c.2168 A > G and c.919–2 A > G mutations, one patient with congenital deafness had m.1555 A > G homoplasmic mutations. Because we did not collect blood samples from the deaf patient's parents, we were unable to accurately determine the inheritance pattern. Studies show that human mitochondrial DNA is maternally inherited47. Therefore, we speculate that the m. 1555A > G mutation originated from the mother. SLC26A4 gene (7q31) has a recessive inheritance48, and pathological mutations of this allele can lead to diseases. Both c.919-2A > G and c.2168A > G are located in SLA26A4. However, this patient had heterozygous mutations at c.919-2A > G and c.2168A > G. Therefore, we speculated that at least one of the parents of this deaf patient carried the G alleles at c.919 and c.2168.
At present, a series of molecular biology methods based on PCR technology are mainly used to detect deafness susceptibility genes, including restriction fragment length polymorphisms, denaturing high-performance liquid chromatography, allele-specific PCR, SNaPshot sequencing, high-throughput sequencing, gene chips, and direct DNA sequencing. WES11 can directly sequence the protein-coding sequence and determine the variation that affects the protein structure; however, it requires expensive sequencing equipment and corresponding analysis software. This method is more suitable for screening many pathogenic genes and identifying new mutation sites without clear diagnostic information. Although microarray technology can detect more deafness-related mutation sites at the same time9, this method also requires a Microarray Scanner and detection system, which increases the cost of detection and can produce false positives and reduce the accuracy of detection49. Sanger sequencing, microarray chips, and WES detection methods also have a common problem: the lid of the reaction tube must be opened to remove the PCR products for follow-up testing, which increases the risk of laboratory contamination. These methods have made great contributions to the discovery and identification of deafness susceptibility genes; however, they have not been widely accepted in clinical practice because they are time-consuming, require expensive equipment and consumables, and cannot detect multiple mutation sites of different genes simultaneously. Deafness susceptibility gene detection can change the traditional passive treatment of deafness diseases into active prevention, which may comprise early detection and intervention and therefore may help prevent deafness. In the present study, 2D-PCR technology was used to detect the genotypes of nine mutation sites in deafness-related genes in a single, closed tube and one-time reaction. This technology amplifies and identifies target genes in batches using a real-time fluorescent quantitative PCR instrument with fluorescent melting curve analysis software. The base-quenching probe used does not require a quenching group, the synthesis of the tag sequence is relatively simple, the reaction reagent is easy to obtain, and the cost is low.
This study was mainly based on the standards of genetic screening for hereditary deafness published in the Chinese Medical Journal in 202150, and the results have reference value for developing genetic testing projects for deafness in Changzhou City, Jiangsu Province. The established 2D-PCR detection technology is simple to operate and rapid, and the detection results at each site do not interfere with each other. Based on the differences in the frequencies of mutation sites in deafness-related genes in different regions, sites that need to be screened can be freely selected to reduce external costs. The limitation of this study is that we have established a 2D-PCR method for detecting nine hotspot mutations in the deafness susceptibility gene, which does not include the 35delG mutation. Considering that the average carrier frequency of the 35delG mutation is highest in Southern Europe and lowest in Eastern Asia51, and that the 35delG mutation is not common among the Chinese population52, the detection method developed in this study is particularly suitable for the Chinese population and not for Southern Europe. Our future goal is to improve and expand the detection range to cover fifteen key hotspot mutations, thereby facilitating genetic screening for deafness across different populations worldwide. In a follow-up study, more mutation sites of deafness-related genes will continue to be optimised, and more comprehensive screening sites will be established to identify the cause as soon as possible. This may aid in scientific prevention, help avoid inducements, delay deafness, and even aid in the selection of scientific treatment methods to fundamentally reduce birth defects.
References
World health organization. Deafness and hearing loss. https://www.who.int/health-topics/hearing-loss#tab=tab_2
Morton, C. C. & Nance, W. E. Newborn hearing screening–a silent revolution. N. Engl. J. Med. 354, 2151–2164. https://doi.org/10.1056/NEJMra050700 (2006).
Fortnum, H. M., Summerfield, A. Q., Marshall, D. H., Davis, A. C. & Bamford, J. M. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ. 323, 536–540 (2001).
De Keulenaer, S. et al. Molecular diagnostics for congenital hearing loss including 15 deafness genes using a next generation sequencing platform. BMC Med. Genomics. 5, 17 (2012).
Sarmadi, A. et al. Whole exome sequencing identifies novel compound heterozygous pathogenic variants in the MYO15A gene leading to autosomal recessive non-syndromic hearing loss. Mol. Biol. Rep. 47, 5355–5364 (2020).
Zhang, Z. et al. A novel variant in MITF in a child from Yunnan-Guizhou Plateau with autosomal dominant inheritance of nonsyndromic hearing loss: A case report Mol. Med. Rep. 17(6054), 6058 (2018).
Yuan, Y. et al. Comprehensive molecular etiology analysis of nonsyndromic hearing impairment from typical areas in China. J Transl Med. 7, 79. https://doi.org/10.1186/1479-5876-7-79 (2009).
Liu, Y. L. et al. Application of the real-time fluorescence PCR melting curve method in gene screening of non-syndromic hearing loss. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 54, 286–291. https://doi.org/10.3760/cma.j.issn.1673-0860.2019.04.009 (2019).
Guomei, C., Luyan, Z., Lingling, D., Chunhong, H. & Shan, C. Concurrent hearing and genetic screening among newborns in Ningbo China. Comput. Math. Methods Med. https://doi.org/10.1155/2022/1713337 (2022).
Li, Y. & Zhu, B. Genotypes and phenotypes of a family with a deaf child carrying combined heterozygous mutations in SLC26A4 and GJB3 genes. Mol. Med. Rep. 14, 319–324. https://doi.org/10.3892/mmr.2016.5280 (2016).
Vallian Broojeni, J., Kazemi, A., Rezaei, H. & Vallian, S. Exome sequencing identifies novel variants associated with non-syndromic hearing loss in the Iranian population. PLoS One. 18, 0289247 (2023).
Hua, S. et al. Influence of APOA5 locus on the treatment efficacy of three statins: Evidence from a randomized pilot study in Chinese subjects. Front. Pharmacol. 9, 352. https://doi.org/10.3389/fphar.2018.00352 (2018).
Yu, Y. et al. Apolipoprotein M gene single nucleotide polymorphisms discovery in patients with chronic obstructive pulmonary disease and determined by the base-quenched probe technique. Gene. 637, 9–13. https://doi.org/10.1016/j.gene.2017.09.029 (2017).
Zhu, Z., Zhang, H., Luo, G., Xu, N. & Pan, Z. Association between the ABCC11 gene polymorphism and the expression of apolipoprotein D by the apocrine glands in axillary osmidrosis. Mol. Med. Rep. 11, 4463–4467. https://doi.org/10.3892/mmr.2015.3274 (2015).
Yang, X. Y. et al. Metallothionein 2A genetic polymorphism and its correlation to coronary heart disease. Eur. Rev. Med. Pharmacol. Sci. 18, 3747–3753 (2014).
Qin, L., Luo, G., Zhang, J. & Xu, N. A novel method of detecting alpha-1 antitrypsin deficiency of Z mutant (GAG(342)AAG) in a single PCR reaction using base-quenched probe. Clin. Chim Acta. 427, 29–33. https://doi.org/10.1016/j.cca.2013.09.042 (2014).
Zheng, L. et al. A novel method of detecting mitochondrial m1494C>T and m1555A>G mutations in a single PCR reaction using base-quenched probe. Clin. Chim Acta. 411, 2114–2116. https://doi.org/10.1016/j.cca.2010.08.040 (2010).
Zhan, Y., Zhang, J., Yao, S. & Luo, G. High-throughput two-dimensional polymerase chain reaction technology. Anal. Chem. 92, 674–682. https://doi.org/10.1021/acs.analchem.9b02030 (2020).
Dai, P. et al. GJB2 mutation spectrum in 2,063 Chinese patients with nonsyndromic hearing impairment. J. Transl. Med. 7, 26. https://doi.org/10.1186/1479-5876-7-26 (2009).
Dai, P. et al. The prevalence of the 235delC GJB2 mutation in a Chinese deaf population. Genet. Med. 9, 283–289. https://doi.org/10.1097/gim.0b013e31804d2371 (2007).
Xiang, Y. B. et al. Mutation analysis of common deafness-causing genes among 506 patients with nonsyndromic hearing loss from Wenzhou city China. Int. J. Pediatr. Otorhinolaryngol. 122, 185–190. https://doi.org/10.1016/j.ijporl.2019.04.024 (2019).
Jiang, Y. et al. Mutation spectrum of common deafness-causing genes in patients with non-syndromic deafness in the Xiamen area China. PLoS One. 10, e0135088. https://doi.org/10.1371/journal.pone.0135088 (2015).
Yuan, Y. et al. Molecular epidemiology and functional assessment of novel allelic variants of SLC26A4 in non-syndromic hearing loss patients with enlarged vestibular aqueduct in China. PLoS One. 7, e49984. https://doi.org/10.1371/journal.pone.0049984 (2012).
Marazita, M. L. et al. Genetic epidemiological studies of early-onset deafness in the US school-age population. Am. J. Med. Genet. 46, 486–491. https://doi.org/10.1002/ajmg.1320460504 (1993).
Report on Prevention and Treatment of Birth Defects in China. National Health Commission of the People’s Republic of China. http://www.gov.cn/gzdt/att/att/site1/20120912/1c6f6506c7f811bacf9301.pdf (2012).
Ambrosi, C. et al. Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix. PLoS One. 8, e70916. https://doi.org/10.1371/journal.pone.0070916 (2013).
Kelsell, D. P. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 387, 80–83. https://doi.org/10.1038/387080a0 (1997).
Estivill, X. et al. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet. 351, 394–398. https://doi.org/10.1016/s0140-6736(97)11124-2 (1998).
Beltramello, M. et al. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem. Biophys. Res. Commun. 305, 1024–1033. https://doi.org/10.1016/s0006-291x(03)00868-4 (2003).
Kecskeméti, N. et al. Analysis of GJB2 mutations and the clinical manifestation in a large Hungarian cohort. Eur. Arch. Otorhinolaryngol. 275, 2441–2448. https://doi.org/10.1007/s00405-018-5083-4 (2018).
Wang, Y. C. et al. Mutations of Cx26 gene (GJB2) for prelingual deafness in Taiwan. Eur. J. Hum. Genet. 10, 495–498. https://doi.org/10.1038/sj.ejhg.5200838 (2002).
Hwa, H. L. et al. Mutation spectrum of the connexin 26 (GJB2) gene in Taiwanese patients with prelingual deafness. Genet. Med. 5, 161–165. https://doi.org/10.1097/01.Gim.0000066796.11916.94 (2003).
Abe, S., Usami, S., Shinkawa, H., Kelley, P. M. & Kimberling, W. J. Prevalent connexin 26 gene (GJB2) mutations in Japanese. J. Med. Genet. 37, 41–43. https://doi.org/10.1136/jmg.37.1.41 (2000).
Park, H. J., Hahn, S. H., Chun, Y. M., Park, K. & Kim, H. N. Connexin26 mutations associated with nonsyndromic hearing loss. Laryngoscope. 110, 1535–1538. https://doi.org/10.1097/00005537-200009000-00023 (2000).
Santoro, M. L., Mobili, L., Mesoraca, A. & Giorlandino, C. First report of prenatal diagnosis of genetic congenital deafness in a routine prenatal genetic test. Prenat. Diagn. 23, 1083–1085. https://doi.org/10.1002/pd.760 (2003).
Bonyadi, M. J., Fotouhi, N. & Esmaeili, M. Spectrum and frequency of GJB2 mutations causing deafness in the northwest of Iran. Int. J. Pediatr. Otorhinolaryngol. 78, 637–640. https://doi.org/10.1016/j.ijporl.2014.01.022 (2014).
Huang, A. et al. Hearing loss associated with an unusual mutation combination in the gap junction beta 2 (GJB2) gene in a Chinese family. Int. J. Pediatr. Otorhinolaryngol. 78, 599–603. https://doi.org/10.1016/j.ijporl.2014.01.008 (2014).
Lu, A. et al. Integrative analyses identified ion channel genes GJB2 and SCNN1B as prognostic biomarkers and therapeutic targets for lung adenocarcinoma. Lung Cancer. 158, 29–39. https://doi.org/10.1016/j.lungcan.2021.06.001 (2021).
Meng, S. et al. The prognostic value and biological significance of gap junction beta protein 2 (GJB2 or Cx26) in cervical cancer. Front Oncol. 12, 907960. https://doi.org/10.3389/fonc.2022.907960 (2022).
Shettar, A., Damineni, S., Mukherjee, G. & Kondaiah, P. Gap junction β-2 expression is negatively associated with the estrogen receptor status in breast cancer tissues and is a regulator of breast tumorigenesis. Oncol. Rep. 40, 3645–3653. https://doi.org/10.3892/or.2018.6764 (2018).
Sun, D., Jin, H., Zhang, J. & Tan, X. Integrated whole genome microarray analysis and immunohistochemical assay identifies COL11A1, GJB2 and CTRL as predictive biomarkers for pancreatic cancer. Cancer Cell Int. 18, 174. https://doi.org/10.1186/s12935-018-0669-x (2018).
Pryor, S. P. et al. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J. Med. Genet. 42, 159–165. https://doi.org/10.1136/jmg.2004.024208 (2005).
Wang, Q. J. et al. A distinct spectrum of SLC26A4 mutations in patients with enlarged vestibular aqueduct in China. Clin. Genet. 72, 245–254. https://doi.org/10.1111/j.1399-0004.2007.00862.x (2007).
Guan, M. X. Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion. 11, 237–245. https://doi.org/10.1016/j.mito.2010.10.006 (2011).
Liu, X. et al. Large-scale screening of mtDNA A1555G mutation in China and its significance in prevention of aminoglycoside antibiotic induced deafness. Zhonghua Yi Xue Za Zhi. 86, 1318–1322 (2006).
Zhu, Y. et al. Mitochondrial haplotype and phenotype of 13 Chinese families may suggest multi-original evolution of mitochondrial C1494T mutation. Mitochondrion. 9, 418–428. https://doi.org/10.1016/j.mito.2009.07.006 (2009).
Giles, R. E., Blanc, H., Cann, H. M. & Wallace, D. C. Maternal inheritance of human mitochondrial DNA. Proc. Natl. Acad. Sci. USA. 77, 6715–6719. https://doi.org/10.1073/pnas.77.11.6715 (1980).
Nonose, R. W. et al. Mutation analysis of SLC26A4 (Pendrin) gene in a Brazilian sample of hearing-impaired subjects. BMC Med. Genet. 19, 73. https://doi.org/10.1186/s12881-018-0585-x (2018).
Zindler, T., Frieling, H., Neyazi, A., Bleich, S. & Friedel, E. Simulating ComBat: how batch correction can lead to the systematic introduction of false positive results in DNA methylation microarray studies. BMC Bioinformatics. 21, 271. https://doi.org/10.1186/s12859-020-03559-6 (2020).
Chinese Multi-center Clinical Research Group on Gene Screening and Diagnosis of Deafness. Genetic Screening Criteria for Hereditary Deafness. Natl. Med. J. China. 101, 97–102. https://doi.org/10.3760/cma.j.cn112137-20201029-02957 (2021).
Mahdieh, N. & Rabbani, B. Statistical study of 35delG mutation of GJB2 gene: a meta-analysis of carrier frequency. Int. J. Audiol. 48, 363–370. https://doi.org/10.1080/14992020802607449 (2009).
Liu, Y., Ke, X., Qi, Y., Li, W. & Zhu, P. Connexin26 gene ( GJB2): prevalence of mutations in the Chinese population. J. Hum. Genet. 47, 688–690. https://doi.org/10.1007/s100380200106 (2002).
Acknowledgements
This work was supported by the Changzhou Municipal Health Commission Youth Talent Science and Technology Project (Grant No. QN202023), and Changzhou Health Healthy Young Talents (Grant No. CZQM2020055) and Leading Talent of Changzhou “The 14th Five-Year Plan” High-Level Health Talents Training Project (grant no. 2022260).
Author information
Authors and Affiliations
Contributions
Y. Y. performed the primer and tag design, data analysis, and drafted the manuscript. J. Z. participated in the tag design and sample collection. Y. Z. participated in the tag design. G. L. designed the study and drafted the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Y., Zhang, J., Zhan, Y. et al. A novel method for detecting nine hotspot mutations of deafness genes in one tube. Sci Rep 14, 454 (2024). https://doi.org/10.1038/s41598-023-50928-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50928-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.