Abstract
Exposure to sustained hypoxia (SH) differentially modifies the hypoxic ventilatory response (HVR) in adults and developing rats. We examined the possibility that postnatal intermittent hypoxia (IH), a more prevalent clinical condition than SH, may lead to significant modifications of ventilatory patterning during development. Sprague-Dawley rat pups were exposed as of the d 1 of life to either SH (10% O2) or IH [alternating room air (RA) and 10% O2 every 90 s] for up to 30 d; controls were exposed to normoxia. HVR (10% O2 for 20 min) was assessed in unrestrained pups at 5, 10, 15, and 30 d of age using whole-body plethysmography. IH pups displayed higher normoxic ventilation (VE) at all ages (p < 0.001 versus control; n = 12 per group), which was not observed in SH animals until 10 d of exposure (p < 0.001 versus control; n = 12 per group). Furthermore, both SH and IH modified properties of peak HVR (pHVR), as well as those of the ensuing hypoxic ventilatory decline (HVD); however, the ventilatory strategies adopted after SH and IH greatly differed. We conclude that both postnatal IH and SH modify normal ventilatory patterning and induce altered HVR, but differ in the ventilatory strategies adopted to mount HVR responses.
Similar content being viewed by others
Main
The mammalian HVR is characteristically biphasic, consisting of initial ventilatory enhancements, termed pHVR, followed by subsequent reductions in ventilatory output, i.e. HVD (1). Ventilatory enhancements during pHVR depend on afferent input from peripheral chemoreceptors, predominantly the carotid bodies, and critically rely on intact glutamatergic signaling in the nucleus of the solitary tract (nTS) (2–5). In contrast, HVD appears to result from complex interactions between both excitatory and inhibitory influences on peripheral chemoreceptors, central respiratory related neurons, and metabolic pathways (1).
The effects of SH exposure on HVR have now been extensively characterized in both adult and developing mammals. In adults, prolonged exposure to SH leads to time-dependent increases in normoxic ventilation with concomitant enhancements of pHVR, a phenomenon that has been termed ventilatory acclimatization to hypoxia (VAH) (6), and mediated through increases in peripheral chemoreceptor sensitivity and centrally mediated adaptations (1). In contrast, when equivalent SH exposures are presented during the immediate postnatal period, progressive blunting of HVR and decreased hypoxic ventilatory sensitivity emerge (7–10). The mechanisms underlying the adaptations observed in the perinatal period are still subject to debate; however, some of the available evidence points to reduced carotid body sensitivity (11–13) and/or changes in neuromuscular transmission, function of respiratory muscles, respiratory mechanics, or feedback control systems (14).
The modification of HVR during the perinatal period is of profound clinical interest given that antecedent hypoxia has been implicated in the pathophysiology of sudden infant death syndrome (SIDS) (15). However, except for high-altitude exposure and environmental conditions of burrowing animals, SH is a relatively unusual occurrence. Indeed, IH is a much more frequent event in clinical settings and accompanies a variety of clinical disorders such as apnea of prematurity and sleep apnea (16).
Prolonged exposure to IH in adult rats elicits progressive enhancements of normoxic ventilatory output, also termed ventilatory adaptation to intermittent hypoxia (VAIH). VAIH is associated with alterations of N-methyl-D-aspartate receptor subtypes in the dorsocaudal brainstem (17) and progressively and rapidly abates with return to preexposure ventilatory levels shortly after termination of the IH exposure. Of note, VAIH differs from the VAH elicited by comparable exposure to SH in adult rats (17). In the developing rat, perinatal exposure to chronic IH leads to VAIH and lifelong modifications of ventilatory control (18). Furthermore, induction of altered normoxic respiration as a result of chronic IH displays an age-dependent pattern (19). However, the consequences of long-lasting IH on the maturation of respiratory control during early postnatal life have not been systematically evaluated. Therefore, we hypothesized that exposure to prolonged IH (up to 30 d), beginning in the immediate postnatal period would lead to modifications of normoxic respiratory characteristics and altered HVR in the developing rat and that such modifications would not completely overlap with those elicited by exposures to SH.
METHODS
Time pregnant Sprague-Dawley rats were purchased from Charles River (Portage, MI), and their male offspring were used for all experiments. Litters were routinely culled to eight pups. The experimental protocols were approved by the Institutional Animal Use and Care Committee and in close agreement with the National Institutes of Health Guide in the Care and Use of Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Experimental protocol.
Male rats were exposed to 30 consecutive days of either SH or IH with their dams starting within the first 12 h of life. As controls, animals were exposed in identical chambers to normoxic conditions with RA being flushed in the chambers in a similar periodicity as that used for IH exposures. At postnatal ages 5, 10, 15, and 30 d, animals were removed from the environmental chambers and respiratory measures were conducted using whole-body plethysmographic techniques after 3–4 h in normoxia.
Hypoxia exposures.
Environmental hypoxic exposures were conducted as previously described (20).
Ventilatory and metabolic recordings.
Respiratory and metabolic measures were continuously acquired in the freely behaving animals using methods previously described (20).
Acute hypoxic ventilatory challenges.
Hypoxic ventilatory challenges were conducted at 5, 10, 15, and 30 d after a normal birth. Animals were weighed and placed in the barometric recording chamber. After stable baseline normoxic values were obtained for at least 30 min, rats were switched to 10% O2–balance N2, using a premixed gas mixture. The acute hypoxic challenge lasted for 20 min followed by 10-min recovery. Ventilatory measures were averaged in 1-min intervals and plotted.
Arterial blood gases.
Arterial blood samples were obtained from implanted arterial catheters in four rats from each group at age 30 d. Catheters (PE-10, Silverwater, B.C., Australia) were placed in the right femoral artery under general anesthesia (Nembutal, 50 mg/kg i.p.) and tunneled subdermally to a second incision just rostral to the scapulae. Measurements were made 3 d later after and consisted in the withdrawal of 75–100 μL of arterial blood after discarding the dead space of the catheter. Samples were immediately analyzed with a blood gas analyzer (ABL 510, Copenhagen, Denmark). Measurements were performed before the hypoxic gas switch and during the hypoxic challenge.
Data analysis.
All values are shown as mean ± standard error (SE) unless indicated otherwise. Baseline ventilatory measures represent the average of at least 10 min of stable normoxic ventilation and were obtained at similar periods of the circadian cycle for all animals. One-way analyses of variance procedures were used to compare differences among outcome variables between the groups at specific time points (i.e. normoxia, pHVR, and HVD) as well as across responses for each experimental group. Significant comparisons were followed by Fisher least significant difference post hoc tests as appropriate. A p value <0.05 was considered to achieve statistical significance for all analyses.
RESULTS
In each group, 12 randomly selected male rats derived from multiple litters were assessed at each time point during the experimental protocol.
Somatic growth.
All litters were weighed shortly after birth (before exposures were initiated) and had similar average weights. Rats exposed to both SH and IH protocols experienced significant growth attenuation in the first 5 d of the exposure compared with RA controls (Fig. 1, p < 0.05); however, SH animals experienced significantly greater growth attenuation than IH animals after 10 and 15 d of exposure compared with controls (Fig. 1, d 10, p < 0.05 versus IH, p < 0.01 versus RA; d 15 p < 0.05 versus IH, p < 0.01 versus RA). However, by d 30 of the protocol, SH showed considerable catch-up growth such that there were no statistical differences between SH and control animals; such growth recovery did not occur in IH-exposed animals, such that at d 30, they weighed significantly less than both SH- and RA-exposed animals (p < 0.01 versus SH and RA). Thus, all measurements of minute ventilation (VE) and tidal volume (VT), which are dependent on body size, were normalized to body weight in grams.
Somatic growth. Average [mean ± standard error of the mean (SEM); n = 12 per group] somatic weights measured at each postnatal age. Rats exposed to both SH (Δ) and IH (○) protocols experienced significant growth attenuation in the first 5 d of the exposure compared with RA controls (▪) (p < 0.05). SH animals experienced significantly greater growth attenuation than IH animals after 10 and 15 d compared with controls (d 10: p < 0.05 vs IH, p < 0.01 vs RA; d 15: p < 0.05 vs IH, p < 0.01 vs RA). At d 30, SH experienced considerable catch-up growth such that there were no statistical differences between SH and controls.
Normoxic VE.
Normoxic VE was significantly increased in IH-exposed rats for all ages (Figs. 2 and 3, p < 0.01 versus RA), whereas normoxic VE in SH-exposed animals only became significantly elevated after 10 d of exposure (p < 0.01 versus RA). Ventilatory measurements were not significantly different between SH and IH rats at d 15; however, SH-exposed animals failed to reach the same level of ventilatory output demonstrated by animals exposed to IH after 30 d. Closer examination of the respiratory components revealed no differences in respiratory frequency among the groups after 5 d of exposure (Fig. 3B); however, after 10 d of exposure, SH animals displayed increased frequency that was significantly greater than in both RA- and IH-exposed animals (p < 0.01 versus both). Enhanced frequency occurred in IH animals only after 15 d of exposure and was not significantly different from SH animals (p < 0.01 IH versus RA; p < 0.01 SH versus RA). After 30 d of exposure, SH animals demonstrated enhanced respiratory frequency that was significantly greater than both RA- and IH-exposed animals (p < 0.001 versus RA; p < 0.001 versus IH). Furthermore, IH-exposed animals displayed increased frequency compared with RA animals (p < 0.01). Closer inspection of the development of normoxic VT (Fig. 3C) revealed that IH-exposed animals displayed enhanced VT compared with RA animals at all time points (p < 0.05), whereas SH-exposed animals demonstrated diminished VT compared with RA after 5 d (p < 0.01) and 30 d (p < 0.01); however, VT was increased after 10 d (p < 0.01) and 15 d (p < 0.01). Mean inspiratory flow (VT/TI, Fig. 3D) was increased at all time points after IH (p < 0.01); however, VT/TI was increased in SH animals only after 10 d of exposure (p < 0.01) and was significantly less elevated than IH animals at d 15. Inspiratory duty cycle (Ti/Ttot, Fig. 3E) was not different from controls in IH animals; however, Ti/Ttot was decreased in SH animals after both 5 and 30 d of exposure (p < 0.01).
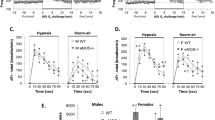
Hypoxic ventilatory responses. Ventilatory measurements (mean ± SEM; n = 12 per group) conducted during acute hypoxic challenges in developing rats exposed to RA (▪), IH (○), or SH (Δ). Data are shown as both absolute minute ventilation (VE) (A, B, C, and D, respectively) and the percentage of change from baseline VE (E, F, G, and H, respectively). For further details, please refer to the text.
pHVR.
Assessment of VE during pHVR revealed that IH-exposed animals exhibited significantly greater ventilatory enhancements than SH-exposed animals at all ages (p < 0.05; Figs. 2 and 4A) and greater enhancements than RA-exposed animals beyond 10 d of exposure (p < 0.01; Figs. 2 and 4A). SH-exposed animals demonstrated significantly increased absolute VE at d 10 (p < 0.01 versus RA) and d 15 (p < 0.01 versus RA) only. However, when presented as the percentage of change from baseline, SH-exposed initially displayed similar pHVR at 5 d (p > 0.05 versus RA; Fig. 4B), which gradually decreased overtime such that after 15 d (p < 0.01 versus RA) and 30 d (p < 0.01 versus RA), relative pHVR was decreased compared with RA-exposed animals. IH-exposed animals exhibited significantly decreased pHVR at only 15 and 30 d (p < 0.01 versus RA) when compared with RA controls. Further examination of ventilatory data revealed no significant differences in respiratory frequency between animals exposed to either SH or IH and RA controls during pHVR after 5 and 10 d of exposure. However, after 15 d, IH-exposed animals exhibited moderately, albeit significantly (p < 0.05 versus RA), enhanced respiratory frequency, whereas after 30 d of exposure, only SH animals demonstrated increased frequency (p < 0.01 versus RA). Relative changes in frequency compared with corresponding antecedent baselines showed that respiratory frequency was greatly increased in RA-exposed pups compared with either IH or SH (p < 0.05; Fig. 4D). Analysis of VT during pHVR revealed a pattern that closely resembled the observations reported above for normoxic VT, whereas relative changes compared with baseline failed to show any significant differences among all three groups (Fig. 4F). Mean inspiratory flow (VT/TI; Fig. 4G) was increased only after 15 and 30 d of IH (p < 0.001); however, VT/TI was increased in SH animals only after 10 and 15 d of exposure (p < 0.01) and was significantly less elevated than IH animals at d 15. Inspiratory duty cycle (Ti/Ttot, Fig. 4H was not different from controls in IH animals; however, Ti/Ttot was decreased in SH animals after both 5 and 30 d of exposure (p < 0.01).
HVD.
Evaluation of HVD revealed attenuation of ventilatory reductions at all ages in IH-exposed animals as expressed in absolute VE (p < 0.01 versus RA; Fig. 5A). By d 10, SH-exposed also revealed significantly attenuated HVD through d 15 (p < 0.01 versus RA). Differences in VE during HVD were brought about increased frequency (Fig. 5C) as well as increased VT (Fig. 5, E and F) in both IH- and SH-exposed animals; however, on d 30, differences in VE were mediated through only enhanced frequency in SH-exposed animals (Fig. 5, C and D). Conversely, when expressed as relative change from baseline VE, HVD was only significantly diminished in SH on d 10 (Fig. 5B) relative to controls, with enhancements in HVD at d 30 for both SH and IH (p < 0.01; Fig. 5B). Mean inspiratory flow (VT/TI, E) was increased at all time points after IH and SH (p < 0.01); however, VT/TI was increased to a greater extent in SH animals after 10 d of exposure (p < 0.01; Fig. 5G). Inspiratory duty cycle (Ti/Ttot, Fig. 5H) was increased in IH animals beginning at d 10 (p < 0.01; Fig. 5H); however, Ti/Ttot was decreased in SH animals after 5 d (p < 0.01) and increased after 15 d of exposure (p < 0.01).
Effect on metabolism.
Metabolic measurements were acquired after 30-d continuous exposure of rats to RA, IH, or SH. No differences emerged among the groups with respect to VCO2 measured during normoxia, pHVR, and HVD (Fig. 6A). However, examination of ventilatory equivalents (VE/VCO2, Fig. 6B) revealed significant enhancements in rats exposed to 30 d of postnatal IH (p < 0.01 versus all other groups; Fig. 6B). No significant differences were found between RA and SH with respect to VE/VCO2.
Metabolic measurements (mean ± SEM; n = 12 per group) acquired after 30 d of exposures of developing rats to RA (▪), IH (○), or SH (Δ). No significant differences emerged among the groups with respect to VCO2 measured during normoxia, pHVR, and HVD (A, p = ns). Further examination of ventilatory equivalents (VE/VCO2, B) revealed significant enhancements in rats exposed at to 30 d of postnatal IH (p < 0.01). No significant differences were found between RA and SH with respect to VE/VCO2.
Arterial blood gases.
Despite significantly altered ventilatory patterns, no significant differences in arterial blood gases emerged among the groups during normoxic conditions or during hypoxic challenges (Table 1). These findings along with the changes in VE/VCO2 described above would suggest substantial alterations in respiratory mechanics in hypoxia-exposed rats.
DISCUSSION
In this study, we found that early postnatal IH and SH induce fundamentally distinct alterations in both normoxic ventilation and ventilatory responses to an acute poikilocapnic hypoxic challenge in the developing rat. Furthermore, early postnatal IH and SH elicit different adaptive respiratory strategies, suggesting the possibility that unique mechanisms may be induced by each respective stimulus.
The effect of SH on respiratory control is certainly one of the most extensively investigated areas in the field. It has been recognized for quite some time that exposure to environmental hypoxia, such as occurs with exposure to high altitude, will lead to time-dependent alterations in control of breathing. In adults, such exposures enhance the response to subsequent acute hypoxic challenges (6,17,21). This enhancement of HVR is associated with increased sensitivity of the peripheral chemoreceptors in combination with centrally mediated adaptations within the excitatory networks underlying HVR (22–24); however, these effects will gradually abate after removal of the hypoxic stimulus. In contrast, perinatal SH exposure will elicit a progressive decreases in hypoxic ventilatory sensitivity and a relative blunting of HVR (7,8,25). Such reductions in HVR may be accounted for in part by reduced O2 sensitivity of the carotid bodies (11–13). Unlike the transient effects observed in adults, the blunted sensitivity to hypoxia associated with perinatal exposure persists into adulthood, suggesting SH-induced developmental changes. In a recent study, hypoxic phrenic responses were intact after perinatal hypoxia, suggesting that the ventilatory plasticity associated with perinatal SH resides downstream to the phrenic motor neuron pool and therefore could be related to neuromuscular transmission, function of respiratory muscles, respiratory mechanics, feedback control, or a combination thereof (14).
Although the current findings are similar to those of other studies of SH in the literature, we should point out that we extended our exposures to 30 d, well beyond the 2 wk previously examined (1). As in such studies, 2 wk of SH led to the anticipated pattern of a relatively blunted pHVR (percentage of change from baseline) and prominent VAH with increased normoxic ventilation attributable to both elevated respiratory rate and enhanced VT. However, after 30 d of postnatal SH, an unexpected and previously unreported catch-up growth and relatively reduced VT were observed. Although VAH was still present after 30 d of postnatal SH, it was primarily ascribable to enhanced respiratory frequency, which is further reflected in a shortened inspiratory duty cycle. Of note is that, despite similarly affected VT and respiratory frequency, a relatively reduced pHVR was still present at this time point. These results may support the conclusions of Bavis et al. (14) in that prolonged SH during development may alter the development of pulmonary mechanics as reflected by the augmentation of VT, although this issue was not specifically addressed in the current study.
The effects of IH on ventilatory control have not been as extensively characterized as those associated with SH. However, incremental evidence is emerging to suggest that IH and SH are indeed very different stimuli that result in unique and differential modifications of respiration. Indeed, studies comparing IH and SH in adult rats revealed markedly different ventilatory adaptations that result in altered normoxic ventilation and HVR (17). Both IH and SH in adult rats were associated with time-dependent ventilatory enhancements and different responses to an acute hypoxic challenge consisting of 10% O2 for 20 min. SH rats demonstrated robust increases in VE during pHVR and a characteristic HVD returning to near baseline normoxic levels; however, IH-exposed animals displayed relative blunting of pHVR compared with control animals and a significantly attenuated HVD. Notably, similar to exposures to SH in the adult rat, the alterations in ventilatory control found in IH animals were completely reversed after 1-mo recovery in normoxia.
Although the effects of IH in the developing mammal to this point had not been systematically characterized, the consequences of relatively shorter exposures (i.e. acute IH) have been reported. Studies conducted in the immediate postnatal period using two different IH protocols in both rabbits [e.g. 10 min 10% O2 followed by 10 min 21% O2, for five cycles; (26)] and in rats [e.g. 21% O2 alternating with 5% O2, nine cycles over 16 h, initiated 6 h after birth (27)] showed significant IH-induced enhancement of normoxic ventilation. Additionally, Peng and colleagues (27) have also shown that IH induces enhancement of HVR, which is correlated with increased sensory output from ex vivo carotid bodies harvested from similarly exposed rat pups. In contrast to these findings, an IH profile consisting of 30 min/d for 6 d attenuated HVR, as demonstrated by diaphragmatic electromyography or as ventilatory output measured using a pneumotachograph expressed as a function of normoxic baseline activity in developing piglets (28,29). Moreover, these findings were associated with increased levels of substance P within the nTS. The latter finding may be of considerable interest given that the receptor for substance P, NK1, has recently been implicated as a critical mediator of HVR (30). Possible explanations for the discrepancies between the findings presented in Peng et al. and Waters et al. may be related to interspecies variability or substantial differences in IH protocols. Indeed, several recent studies have examined the effects of varying durations and severity of IH profiles (31–34). The overwhelming consensus reached by these studies is that the presentation and profile of the IH exposure is directly related to physiologic responses elicited. Therefore, it is necessary to consider inherent differences between IH profiles when trying to draw comparisons between studies.
The findings of the present study demonstrate that developmental IH elicits ventilatory adaptations that are remarkably different from those observed after SH. Indeed, IH uniquely modified both normoxic ventilation and HVR in developing rat pups through specific alterations in respiratory frequency and VT. Furthermore, these variations do not appear to be related to alterations in metabolic rate and metabolic responses to hypoxia as reflected by VCO2 measurements (Fig. 5). In addition, ventilatory disparities between the groups could not be accounted for by variations in arterial blood gases despite the substantial differences in minute ventilation observed during normoxia and during hypoxic challenges (Table 1). Taken together, this evidence could suggest modification of both peripheral (13) and central pathways underlying ventilatory rhythmogenesis (35); however, at this point, based on the discrepancies in ventilatory measurements associated with SH and particularly with IH, we tend to favor the possibility that altered respiratory mechanics associated with hypoxic exposure and leading to increased dead space constitute the major contributors to altered respiratory output. Indeed, further studies will be required to identify the mechanism(s) underlying chronic IH-induced ventilatory plasticity and potentially the structural remodeling changes in lung parenchyma that may be associated with this hypoxic paradigm at an early postnatal age.
In summary, prolonged exposure to either postnatal SH and IH will elicit remarkable, albeit unique, consequences on respiratory development. Furthermore, the consequences of early postnatal exposure to IH are of profound clinical importance given the high prevalence of sleep-disordered breathing in infants and the association of antecedent hypoxia with SIDS (15). Therefore, future investigations will be critical to delineate the molecular and cellular changes underlying the unique adaptive phenomena associated with IH during development.
Abbreviations
- HVD:
-
hypoxic ventilatory depression
- HVR:
-
hypoxic ventilatory response
- IH:
-
intermittent hypoxia
- nTS:
-
nucleus tractus solitarii
- pHVR:
-
peak hypoxic ventilatory response
- SH:
-
sustained hypoxia
- VAH:
-
ventilatory acclimatization to hypoxia
- VAIH:
-
ventilatory adaptation to intermittent hypoxia
References
Powell FL, Milsom WK, Mitchell GS 1998 Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134
Lin J, Suguihara C, Huang J, Hehre D, Devia C, Bancalari E 1996 Effect of N-methyl-D-aspartate-receptor blockade on hypoxic ventilatory response in unanesthetized piglets. J Appl Physiol 80: 1759–1763
Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K 1994 In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol 478: 55–66
Soto-Arape I, Burton MD, Kazemi H 1995 Central amino acid neurotransmitters and the hypoxic ventilatory response. Am J Respir Crit Care Med 151: 1113–1120
Ohtake PJ, Torres JE, Gozal YM, Graff GR, Gozal D 1998 NMDA receptors mediate peripheral chemoreceptor afferent input in the conscious rat. J Appl Physiol 84: 853–861
Aaron EA, Powell FL 1993 Effect of chronic hypoxia on hypoxic ventilatory response in awake rats. J Appl Physiol 74: 1635–1640
Mortola JP, Morgan CA, Virgona V 1986 Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol 61: 1329–1336
Eden GJ, Hanson MA 1987 Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol 392: 11–19
Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, Thoren HP, Lagercrantz H 2002 Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Arch 443: 858–865
Peyronnet J, Roux JC, Geloen A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y 2000 Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol 524: 525–537
Sladek M, Parker RA, Grogaard JB, Sundell HW 1993 Long-lasting effect of prolonged hypoxemia after birth on the immediate ventilatory response to changes in arterial partial pressure of oxygen in young lambs. Pediatr Res 34: 821–828
Jackson A, Nurse C 1995 Plasticity in cultured carotid body chemoreceptors: environmental modulation of GAP-43 and neurofilament. J Neurobiol 26: 485–496
Sterni LM, Bamford OS, Wasicko MJ, Carroll JL 1999 Chronic hypoxia abolished the postnatal increase in carotid body type I cell sensitivity to hypoxia. Am J Physiol 277: L645–L652
Bavis RW, Olson EB Jr, Vidruk EH, Fuller DD, Mitchell GS 2004 Developmental plasticity of the hypoxic ventilatory response in rats induced by neonatal hypoxia. J Physiol 557: 645–660
Jones KL, Krous HF, Nadeau J, Blackbourne B, Zielke HR, Gozal D 2003 Vascular endothelial growth factor in the cerebrospinal fluid of infants who died of sudden infant death syndrome: evidence for antecedent hypoxia. Pediatrics 111: 358–363
Gozal D, Lipton AJ, Jones KL 2002 Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep 25: 59–65
Reeves SR, Gozal E, Guo SZ, Sachleben LR Jr, Brittian KR, Lipton AJ, Gozal D 2003 Effect of long-term intermittent and sustained hypoxia on hypoxic ventilatory and metabolic responses in the adult rat. J Appl Physiol 95: 1767–1774
Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ 2003 Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med 167: 1540–1547
Reeves SR, Gozal D 2005 Changes in ventilatory adaptations associated with long-term intermittent hypoxia across the age spectrum in the rat. Respir Physiol Neurobiol
Reeves SR, Gozal D 2006 Changes in ventilatory adaptations associated with long-term intermittent hypoxia across the age spectrum in the rat. Respir Physiol Neurobiol 150: 135–143
Dwinell MR, Powell FL 1999 Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J Appl Physiol 87: 817–823
Gamboa A, Leon-Velarde F, Rivera-Ch M, Palacios JA, Pragnell TR, O'Connor DF, Robbins PA 2003 Selected contribution: acute and sustained ventilatory responses to hypoxia in high-altitude natives living at sea level. J Appl Physiol 94: 1255–1262
Leon-Velarde F, Gamboa A, Rivera-Ch M, Palacios JA, Robbins PA 2003 Selected contribution: peripheral chemoreflex function in high-altitude natives and patients with chronic mountain sickness. J Appl Physiol 94: 1269–1278
Alea OA, Czapla MA, Lasky JA, Simakajornboon N, Gozal E, Gozal D 2000 PDGF-beta receptor expression and ventilatory acclimatization to hypoxia in the rat. Am J Physiol Regul Integr Comp Physiol 279: R1625–R1633
Frappell PB, Mortola JP 1994 Hamsters vs. rats: metabolic and ventilatory response to development in chronic hypoxia. J Appl Physiol 77: 2748–2752
Trippenbach T 1994 Ventilatory and metabolic effects of repeated hypoxia in conscious newborn rabbits. Am J Physiol 266: R1584–R1590
Peng YJ, Rennison J, Prabhakar NR 2004 Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol 97: 2020–2025
Waters KA, Laferriere A, Paquette J, Goodyer C, Moss IR 1997 Curtailed respiration by repeated vs. isolated hypoxia in maturing piglets is unrelated to NTS ME or SP levels. J Appl Physiol 83: 522–529
Waters KA, Tinworth KD 2001 Depression of ventilatory responses after daily, cyclic hypercapnic hypoxia in piglets. J Appl Physiol 90: 1065–1073
Wickstrom HR, Berner J, Holgert H, Hokfelt T, Lagercrantz H 2004 Hypoxic response in newborn rat is attenuated by neurokinin-1 receptor blockade. Respir Physiol Neurobiol 140: 19–31
Gozal D, Gozal E 1999 Episodic hypoxia enhances late hypoxic ventilation in developing rat: putative role of neuronal NO synthase. Am J Physiol 276: R17–R22
Peng YJ, Prabhakar NR 2004 Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96: 1236–1242
Waters KA, Gozal D 2003 Responses to hypoxia during early development. Respir Physiol Neurobiol 136: 115–129
McGuire M, Zhang Y, White DP, Ling L 2002 Effect of hypoxic episode number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol 93: 2155–2161
Reeves SR, Guo SZ, Brittian KR, Row BW, Gozal D 2006 Anatomical changes in selected cardio-respiratory brainstem nuclei following early postnatal chronic intermittent hypoxia. Neurosci Lett 402: 233–237
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by National Institutes of Health grant HL69932, The Children's Foundation Endowment for Sleep and Neurobiology Research, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund. S.R.R. is the recipient of predoctoral fellowships from the Ohio Valley Chapter of the American Heart Association and National Institutes of Health (F30 NS-48770).
Rights and permissions
About this article
Cite this article
Reeves, S., Gozal, D. Respiratory and Metabolic Responses to Early Postnatal Chronic Intermittent Hypoxia and Sustained Hypoxia in the Developing Rat. Pediatr Res 60, 680–686 (2006). https://doi.org/10.1203/01.pdr.0000246073.95911.18
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000246073.95911.18