Abstract
In this study we wanted to assess the relationship between mixed venous oxygen saturation (Svo2) and tissue oxygenation. For that, we compared the values of Svo2 with oxygen delivery (Do2), oxygen consumption (Vo2), and markers of tissue hypoxia such as lactate and pyruvate during progressive hypoxemia. Eight 10-14-d-old piglets were anesthetized, tracheotomized, intubated, and ventilated. A fiberoptic catheter was placed in the carotid artery to monitor arterial oxygen saturation(Sao2). A thoracotomy was performed, and a fiberoptic catheter was placed in the pulmonary artery to monitor Svo2. A transit time ultrasound flow probe was positioned around the ascending aorta to measure aorta flow. Progressive graded hypoxemia was induced by decreasing fractional inspiratory oxygen concentration (Fio2) from 1.0 to 0.30, 0.21, 0.15, and 0.10. After each Fio2 interval blood samples were taken for blood gases, lactate, and pyruvate. Do2 and Vo2 were calculated. Svo2 decreased similarly to Sao2. A value of Svo2 of more than 40% excluded oxygen restricted metabolism. When Do2 decreased below a critical range (8.4-12.8 mL/kg·min), Svo2 decreased below 15%, and lactate and the lactate/pyruvate ratio increased. We conclude1) that baseline Svo2 values excluded oxygen-restricted metabolism, 2) that Svo2 values between 15 and 40% were not a marker for oxygen-restricted metabolism, and 3) that Svo2 values below 15% were associated with oxygen-restricted metabolism. Reduced Svo2 values must be interpreted as a change of the factors that determine the balance between Do2 and Vo2 and as a warning that, with further reduction of Svo2, oxygen restricted metabolism can develop.
Similar content being viewed by others
Main
Svo2 reflects the residual oxygen after tissue oxygen extraction and represents the combined sufficiency of Cao2, Qt, and tissue Vo2(1). In adult intensive care, Svo2 has been used to monitor cardiac output. In neonatal medicine, Svo2 monitoring is used during extracorporeal support to monitor the sufficiency of Do2(2). Continuous Svo2 measurement was proposed in neonatal intensive care to monitor the oxygen sufficiency at the tissue level, but it is only used occasionally(1, 3).
We have determined the feasibility and the accuracy of a fiberoptic catheter system in the newborn, providing a continuous central Svo2 measurement in the right atrium(4). We have also described Svo2 values in 1-7-d-old preterm infants breathing room air(5). However, critical values of Svo2, below which oxygen sufficiency at the tissue level is threatened, have not been properly evaluated in the newborn period.
It is known, that, when oxygen delivery at the tissue level is insufficient, oxygen-restricted metabolism will develop and the concentration of biochemical markers of tissue hypoxia such as lactate and pyruvate will appear in increased concentrations in the blood(6, 7). In this study we attempted to assess the relation between Svo2 and tissue oxygen sufficiency. Hence, we examined the values of Svo2 in comparison with Do2, Vo2, and biochemical markers of tissue hypoxia to determine a normal and safe range of Svo2 and to determine a critical value of Svo2 below which oxygen restricted metabolism will develop.
METHODS
This study was approved by the animal care committee of the University of Maastricht.
Animal preparation. Eight piglets between 10 and 14 d of age were anesthetized using halothane for induction, tracheotomized, intubated, and ventilated with room air. Anesthesia was maintained by continuous i.v. infusion of pentobarbital sodium and pancuronium in glucose 10% as required. A 4 Fr fiberoptic catheter (Abbott Laboratories, Chicago IL) was placed in the right carotid artery for continuous monitoring of Sao2, for blood sampling, and for monitoring arterial blood pressure. A 5 Fr catheter was placed in the jugular or femoral vein for fluid administration. A thoracotomy was performed, and the ductus arteriosus was ligated. A transit time ultrasound blood volume flow probe (Transonic System Inc., Ithaca, NY) was positioned around the ascending aorta to monitor continuously aorta blood flow. The pulmonary artery was catheterized with a 4 Fr fiberoptic catheter (Abbott Laboratories) for monitoring Svo2 continuously and for blood sampling. A rectal probe was placed to monitor core body temperature.
Protocol. The piglets were ventilated to maintain Paco2 at 5.0 ± 1.0 kPa with a pressure-limited, time-cycled ventilator (Bourns BP-200, Medical Systems, Inc., Riverside, CA). Inspiratory gas was warmed and humidified. We induced progressive graded hypoxemia by changing the proportion of oxygen/nitrogen in the inspiratory gas resulting in a Fio2 of 1.0, 0.30, 0.21, 0.15, and 0.10. Every fixed Fio2 was kept for an interval of 15 min. When blood pressure dropped or bradycardia occurred at the lowest Fio2, the interval was shortened or discontinued. At the end of each interval blood samples were taken simultaneously from the carotid artery for blood gases, Hb, lactate, and pyruvate and from the pulmonary artery for blood gases and Hb. The study included two series of the different Fio2 intervals with a 1-h period between for recovery. We have repeated the series of graded hypoxia, because all animals except one regained a stable condition after the first series of the Fio2 intervals and because more data could be obtained per animal. A blood transfusion was given for accidental blood loss during the operative procedure or when the Hb level decreased, due to blood sampling during the experiment, to maintain a baseline Hb level of 5.0 ± 1.0 mmol/L.
Measurements. The fiberoptic catheter system(Oximetrix System, Abbott Laboratories) includes a fiberoptic catheter and a microprocessor-based oximeter. The light back-scattered from the oxygenated and deoxygenated blood is transmitted by the receiving fiberoptic bundle to a photodetector. The oximeter computes percentage of oxygen saturation values based on the electrical signals from the photodetector.
Sao2, Svo2, arterial blood pressure, blood flow in the aorta, heart rate, and core body temperature were sampled every 10 s, displayed, and stored by a software system (Hemodynamic Data Acquiring System (HDAS) developed at the Service Center, University of Maastricht). Blood samples for blood gases were analyzed on an ABL 3 acid-base analyzer (Radiometer, Copenhagen, Denmark) corrected to the animal core body temperature. Sao2, arterial Hb (mmol/L), and Svo2 were analyzed on an OSM2(Radiometer, Copenhagen, Denmark). Lactate and pyruvate were analyzed in arterial blood samples taken simultaneously with the samples for acid-base analysis. One milliliter of arterial blood was pipetted into a tube containing 1 mL of 1.2 M perchloric acid to obtain a protein-free solution. The enzyme lactate dehydrogenase added to this solution, catalyzes the conversion of pyruvic acid in the presence of NADH to lactic acid and catalyzes the conversion from lactic acid to pyruvic acid in the presence of NAD. The reduced or increased absorbance at 340 nm becomes a measure of the amount of pyruvic acid and of lactic acid, respectively(8).
Calculations. Cao2 and Cvo2 were calculated using the equations
Cao2 (mL/dL) = (1.36 × Sao2 × Hg (mmol/L) × 1.611) + (0.0031 × Pao2(kPa) × 7.5)
and
Cvo2 (mL/dL) = (1.36 × Svo2 × Hg (mmol/L) × 1.611 + (0.0031 × Pvo2 (kPa) × 7.5)
respectively.
Qt was determined by the mean value of the blood flow in the aorta during the last 5 min of each Fio2 interval measured by the flow sensor.
Do2 was calculated from its definition:
Do2 (mL/kg·min) = Cao2 (mL/dL) × Qt(mL/kg·min) / 100.
Vo2 was calculated using the Fick equation:
Vo2 (mL/kg·min) = Qt / 100 × (Cao2 - Cvo2).
Fractional oxygen extraction was calculated as the Vo2 divided by Do2.
P50 was calculated from venous blood gas samples using Svo2 and Pvo2 as has been described in the literature(9). O2 debt was calculated by multiplying the difference between the mean baseline Vo2 at Fio2 = 1.0 and the estimated Vo2 in the Fio2 interval by the duration of that same Fio2 interval. Cumulative O2 debt was calculated by summing up the O2 debt of each interval. The change of arterial pH, Paco2 Pao2, Pvo2, P50, Hb, temperature, heart rate, Qt, lactate, pyruvate, lactate/pyruvate ratio, Vo2 C(a-v)o2, and fractional oxygen extraction was calculated in comparison with the mean baseline value of these items at Fio2 = 1.0 in each series of Fio2 intervals in each individual animal. Absolute values were used for Sao2, Svo2, and Do2.
Data analysis. Mean, SD, median value, and percentile were calculated by descriptive statistical methods. Data are presented as mean± SD. Data at the different Fio2 intervals are presented as absolute values and as a mean change in comparison with the mean baseline values at Fio2 = 1.0 in each individual animal. To test whether a difference existed between the two series of induced hypoxemia, a Mann-WhitneyU test was performed for each Fio2 interval. Baseline data were compared with the data at the different low Fio2 intervals using one-way ANOVA and contrasted according to Tukey B method.
To determine the critical value of Do2, that is the Do2 below which the change in lactate concentration increased rapidly, we adapted a technique from Mellits(10) and Fahey and Lister(11) to find the section point of two lines of regression. This included constructing the best pair of regression lines that included all points only once. The best pair of regression lines was defined as to minimize the total of the residual sum of squares of each line in the pair. Their intersection point was determined by solving the equations of the pair of regression lines. The x coordinate of the intersection point is the critical Do2(11). This technique was also applied to change in lactate/pyruvate ratio and change in Vo2versus Do2.
To test whether a value of Svo2 was predictive for a change in lactate, a change in lactate/pyruvate ratio and a change in Vo2, a 2× 2 contingency table was derived for each item, and sensitivity, specificity, and positive predictive accuracy were calculated. Tests were considered to be significant when p ≤ 0.05.
RESULTS
Eight piglets were studied with a mean weight of 4.08 ± 0.8 kg and at a mean age of 11 ± 2 d. In seven piglets a complete protocol of two series of experiments could be performed. No significant differences were found between the results of corresponding Fio2 intervals in the two consecutive series of induced hypoxemia. Measured and calculated data during the five Fio2 intervals are shown in Table 1. Baseline values regarding Qt, calculated Vo2, Hb, Cao2, and P50 were consistent with values in 10-14-d-old piglets, described by others(12, 13).
Hemodynamic responses during the different intervals consisted of an increased heart rate in the last two Fio2 intervals and a decreased blood pressure at the last interval, whereas cardiac output remained unaltered(Table 1).
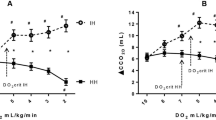
Progressive graded hypoxemia induced a decrease in Sao2 and Pao2 from 98.0 to 24.7% and from and 51.4 to 2.7 kPa, respectively. Svo2 decreased similarly to the decreasing Sao2(Fig. 1). Therefore, in all but the lowest Fio2 interval the difference between arterial and venous oxygen content C(a-v)o2 remained unaltered, and a constant Vo2 was preserved(Table 1). In the lowest Fio2 interval Do2 decreased further, and Vo2 decreased significantly(Table 1, Fig. 1).
Sao2, Svo2, Do2, and change Vo2 in relation to the different Fio2 intervals. Note that Svo2 decreased similarly to Sao2; change in Vo2 was constant in all but the last interval. In the lowest Fio2 interval Do2 further decreased, and change Vo2 was significantly reduced(*). Data are presented as mean ± SD.
Lactate and pyruvate during baseline showed a broad dispersion of values reflecting different postoperative status (accidental blood loss, intubation difficulties, and so forth). The change in lactate and lactate/pyruvate ratio was significant in the last Fio2 interval. Pyruvate change was not significant from the baseline value (Table 1).
The relationships between Do2 and change in lactate, change in the lactate/pyruvate ratio, and change in Vo2 were analyzed inFigure 2 according to the technique from Mellits(10). The critical Do2 value was 12.86 mL/kg·min for lactate, 8.43 mL/kg·min for lactate/pyruvate ratio, and 11.82 mL/kg·min for Vo2. Above the critical Do2, the change of lactate, lactate/pyruvate ratio, and Vo2 were only slight dependent on Do2. Below the critical Do2 a significant regression coefficient (slope) was obtained.
Linear regression analysis showed a good correlation between Svo2 in relation with Do2 (r = 0.87; p < 0.0001). When Do2 decreased below the critical range (8.43 and 12.86 mL/kg·min), Svo2 values less than 15% were observed(Fig. 3).
Svo2 in relation with Do2. The regression line with confidence intervals is: 2.51 × Do2 -4.01 (r= 0.87; p < 0.0001). Vertical lines represent critical Do2 calculated for change lactate/pyruvate ratio and change lactate, respectively. Below this critical range of Do2 (8.43-12.86 mL/kg·min) Svo2 values of less than 15% were observed.
In Figure 4 the relationship between Svo2 and the change in lactate, the change in lactate/pyruvate ratio, and the change in Vo2 was examined. We considered a change in lactate of more than 1.03 mmol/L, a change in lactate/pyruvate ratio of more than 2.45, and a change in Vo2 of less than -2.25 mL/kg·min as significant, because these values represented the mean change ± 2 SD at Fio2 = 1.00. Svo2 values of more than 40% were considered as baseline, as it represented the mean value ± 2 SD in the highest Fio2 interval. Svo2 values of less than 15% were considered critical values, as they represented the values encountered below the critical range of Do2. Lactate, lactate/pyruvate ratio, and Vo2 remained mainly unaltered until Svo2 was less than 15%.
Change in lactate, change in the lactate/pyruvate ratio, and change in Vo2 in relation to Svo2. Vertical broken lines indicate a Svo2 value of 15% (mean ± 2 SD at Fio2 = 0.10) and a Svo2 value of 40% (mean ± 2 SD at Fio2 = 1.00). The horizontal broken lines indicate a change in lactate of 1.03 mmol/L (mean change ± 2 SD at Fio22 = 1.0), change in the lactate/pyruvate ratio of 2.45 (mean change ± 2 SD at Fio2 = 1.0), and change in Vo2 of -2.25 mL/kg·min (mean change ± 2 SD at Fio2 = 1.0). Note that lactate, the lactate/pyruvate ratio, and Vo2 significantly change when Svo2 values below 15% are encountered.
To test whether values of Svo2 above or below 40% were predictive of a significant change in lactate, in lactate/pyruvate ratio, and in Vo2, a 2 × 2 contingency table was derived for each item; a change in lactate of ≥1.03 mmol/L and of <1.03 mmol/L, a change in lactate/pyruvate ratio of ≥2.45 and of <2.45, and a change in Vo2 of > -2.25 mL/kg·min and ≤-2.25 mL/kg min constituted the columns, and Svo2 values above 40% and below 40% were used for the rows. A value of Svo2 above 40% was 68% sensitive and 92% specific for a change within baseline ± 2 SD in lactate concentration and 79% sensitive and 96% specific for a change in lactate/pyruvate ratio and 63% sensitive and 94% specific for a change Vo2 within baseline ± 2 SD. The positive predictive accuracy of Svo2 above 40% was 94, 97, and 98% for a change in lactate, lactate/pyruvate ratio, and Vo2 within baseline ± 2 SD. However, the positive predictive accuracy of a Svo2 below 40% for a significant change above baseline ± 2 SD was only 57, 76, and 37%.
In the same way we tested whether Svo2 values below or above 15% were predictive of a significant change in lactate, lactate/pyruvate ratio, and Vo2. A value of Svo2 below 15% was 84% sensitive and 49% specific for a significant increase in lactate, 77% sensitive and 100% specific for a significant increase in lactate/pyruvate ratio, and 84% sensitive and 96% specific for a change in Vo2. The positive predictive accuracy of Svo2 below 15% was 87, 100, and 84% for a significant change in lactate, lactate/pyruvate ratio, and Vo2. The positive predictive accuracy of a Svo2 above 15% for a change within baseline± 2 SD was 92, 86, and 96%. The positive predictive accuracy of a Svo2 between 15 and 40% for a significant change from baseline ± 2 SD was 13, 60, and 12%.
In other words, Svo2 values above 40% were very frequently associated with baseline lactate, lactate/pyruvate ratio, and baseline Vo2. Svo2 values below 15% were very frequently associated with increased lactate, lactate/pyruvate ratio and decreased Vo2. Svo2 values between 15 and 40% were very occasionally associated with an increased lactate, lactate/pyruvate ratio, and decreased Vo2.
DISCUSSION
We assessed the relationship between Svo2 and tissue oxygenation in piglets by inducing graded progressive hypoxemia. The main results of this study were: 1) Svo2 values of more than 40% excluded oxygen-restricted metabolism, 2) Svo2 values of less than 15% were associated with oxygen-restricted metabolism, and 3) Svo2 values between 15 and 40% were very frequently associated with normal tissue oxygen metabolism. The aim of this study was neither to determine the value of Svo2 measurement in neonatal intensive care medicine, nor to describe the limits of the range of Svo2 values for direct use in neonatal intensive care. The critical values of 15 and 40% found in this study cannot be extrapolated to patient care in a neonatal intensive care unit.
This hypoxia model consists of progressively graded hypoxemia by reducing Fio2 to reduce Do2. In our study the circulatory adjustments as increased cardiac output to compensate for a decreased Cao2 were variable, as it is known from the literature(14). Microcirculatory adjustments were not measured in this study, but may affect the actual oxygen flow from the capillary bed to the cells and may consequently affect the Svo2 value. As other types of hypoxia (anemic hypoxia, circulatory failure hypoxia), could have other patterns of circulatory adjustments and compensations, one must be cautious to extrapolate the findings from this hypoxia model to other types of hypoxia.
The series of graded hypoxemia were started with hyperoxia in the Fio2 = 1.0 interval with a wide variation in baseline conditions, probably due to animal preparation. This is also illustrated by the fact that in the third Fio2 = 0.21 interval a mean Sao2 value of 82% was present. However, these variations in baseline conditions did not affect the changes induced by the different Fio2 intervals: Sao2, the main factor of Cao2, decreased significantly in the Fio2 intervals, resulting in a significant reduction of Do2. Svo2 decreased similarly with Sao2, which maintained a stable difference between arterial and venous oxygen content [C(a-v)o2] and preserved a constant Vo2 in all but the last Fio2 interval (Fig. 1). When Fio2 further decreased, C(a-v)o2 decreased and Vo2 was reduced with the development of oxygen restricted metabolism and lactic acidosis. The initial reduction of Svo2 was not accompanied by a reduction of Vo2 or an increased concentration of lactate. Only with a severe reduction of Svo2 below 15%, regardless of the basal condition of the animal, lactate increased and Vo2 decreased.
In our study Svo2 values above 15% were found with only slight changes in lactate concentration, in lactate/pyruvate ratio and in Vo2. In adult intensive care medicine, tolerance of unusually low Svo2 values have been described(15). Therefore, the reduction of Svo2 per se is not an indication of oxygen-restricted metabolism and lactic acidosis. However, in some instances in our study Svo2 values above 15% were associated with oxygen-restricted metabolism and lactic acidosis. Because Svo2 reflects only the global tissue oxygen extraction, moderately reduced Svo2 values with a normal C(a-v)o2 cannot exclude a reduced Vo2 in individual organs, primarily in organs with a high oxygen extraction, like the myocardium and the brain(16, 17). In accordance with this we found that the critical Do2 calculated for change in lactate concentration was higher than the critical Do2 calculated for change in Vo2. This supports the hypothesis that oxygen-restricted metabolism in individual organs could initiate before a low Svo2 value is observed and global Vo2 is reduced.
In adult intensive care medicine the relation between Svo2 and lactic acidosis was studied in relation to prognosis and mortality. Astizet al.(18) failed to identify a threshold value for Svo2 below which lactate increased. Lactic acidosis developed over a wide range of both Do2 and Svo2. In a retrospecttive analysis of critical ill patients Rashkin et al.(19) showed that mixed venous oxygen tension was not a reliable indicator of blood lactate. Thus, a low Svo2 value can be associated with both a normal tissue oxygen metabolism and an oxygen-restricted tissue metabolism. In our study only Svo2 values below 15% were very frequently associated with oxygen-restricted metabolism. However, these findings do not imply that Svo2 is not useful in the estimation of tissue oxygenation. We found that Svo2 values of more than 40% (mean ± 2 SD at Fio2 = 1.0) were not associated with oxygen-restricted metabolism. Therefore, this range of Svo2 values can be interpreted as an indication of normal tissue oxygenation, i.e. a normal balance between tissue oxygen delivery and demand. However, even normal and high Svo2 values have been associated with lactic acidosis in diseases with disturbances of the microcirculation with precapillary shunting and inability of the tissues to extract enough oxygen, like sepsis and adult respiratory distress syndrome(15, 20). This was not the case in our study.
Not only the reduction of Do2 and Vo2, but also the duration and cumulation of the effects of the different Fio2 intervals must be considered. The cumulation of the oxygen debt in the last three Fio2 intervals can be a reason for the increased lactate concentration in the last Fio2 interval. Cumulative O2 debt was calculated, and the correlation between cumulative O2 debt and lactate and lactate/pyruvate ratio was analyzed to control for the cumulative effect of different Fio2 intervals. Cumulative O2 debt was linearly related to the change in lactate and in lactate/pyruvate ratio, but the correlation coefficient (r = 0.53; r = 0.70) was not different from the correlation coefficient between the change in Vo2 and the change in lactate and in lactate/pyruvate ratio (r = 0.53; r = 0.53). The linear correlation analysis between the change in Vo2 and cumulative O2 debt yielded a good correlation (r = 0.69,p < 0.001). Cumulative O2 debt was in this hypoxic piglet model not a better parameter of oxygen restricted metabolism than change in Vo2. Additionally no differences were found in changes in lactate concentration and in lactate/pyruvate ratio between the two series of Fio2 intervals.
In this study Vo2 was calculated according to Fick's principle and it was dependent on the precision of Cao2, Cvo2, and of cardiac output measurements. The Svo2 values are reliable, because after closure of the ductus arteriosus during the procedure, blood samples and fiberoptic catheter readings reflect truly oxygen saturation values of the mixed venous blood. Whereas oxygen saturation measurements and oxygen content are reliable, the different methods of measurement of cardiac output have many sources of error(21). The value of calculated Vo2 has been questioned(22). In particular when calculated Do2 and calculated Vo2 are compared as independent and dependent variables and a pathologic dependence of Vo2 on Do2 was demonstrated, erroneous mathematical coupling of shared variables was suggested(23). In our study cardiac output was measured directly by a 7-mm transit time ultrasonic flow transducer positioned around the aorta. The transducer was precalibrated in vitro. This transit time ultrasonic technique is described as more precise and as easier to apply than formerly used techniques(24). The transit time flow measurements were used as a“gold standard” and confirmed to be accurate compared with a radionuclide-labeled microsphere technique(25). Therefore, we considered the calculated Do2 and Vo2, derived from the mentioned data, both reliable and accurate.
The biochemical markers lactate and pyruvate were used as the indicators for oxygen-restricted metabolism. The use of lactate as an index for tissue oxygen sufficiency has several limitations. Lactic acidosis originates only when lactate production exceeds hepatic, renal, and muscle lactate uptake(7). Thus, the lactate value is among others dependent on the capacity of liver and kidney to extract and use lactate(6). The lactate/pyruvate ratio was measured to estimate the ratio between the reduced and oxidized molecules in the cytosol; this intracellular redox state is dependent upon oxidative function in the mitochondrion(26) and consequently upon an adequate O2 supply to the mitochondrion(27). In contrast, an increase of lactate and pyruvate without a change in lactate/pyruvate ratio could be a result of inactivation or down-regulation of the pyruvate dehydrogenase complex, which controls the entry of two carbon fragments from pyruvate into the citric acid cycle(28). It was suggested that the measurement of lactate/pyruvate ratio would discriminate between hypoxic and nonhypoxic induced changes of lactate(28).
We conclude that in this hypoxia model, 1) Svo2 values above 40% excluded oxygen-restricted metabolism, 2) that Svo2 values between 15 and 40% in general were not associated with oxygen-restricted metabolism or lactic acidosis, and 3) that values of Svo2 below 15% were associated with oxygen-restricted metabolism. When Svo2 is used in patient care, reduction of Svo2 must be interpreted as a reduction of the residual oxygen after tissue oxygen extraction without necessarily affecting Vo2 and without a definite indication of lactic acidosis. Reduced Svo2 values must be interpreted as a change of the factors that determine the balance between Do2 and Vo2 and as a warning that, with further reduction of Svo2, oxygen-restricted metabolism can develop.
Abbreviations
- Svo2 :
-
mixed venous O2 saturation
- Sao2 :
-
arterial O2 saturation
- Do2 :
-
O2 delivery
- Vo2 :
-
O2 consumption
- Fio2 :
-
fractional inspiratory O2 concentration
- Qt:
-
cardiac output
- Cao2 :
-
arterial O2 content
- Cvo2 :
-
venous O2 content
- C(a-v)o2:
-
difference between arterial and venous O2 content
- Pao2 :
-
arterial partial pressure of O2
- Pvo2 :
-
mixed venous partial pressure of O2
- P50 :
-
partial pressure of O2 at 50% O2 concentration
- Paco2 :
-
arterial partial pressure of CO2(kPa)
- Pao:
-
mean aortal blood pressure (mm Hg)
References
O'Connor TA, Hall RT 1994; Mixed venous oxygenation in critically ill neonates. Crit Care Med 22: 343–346.
Bartlett RH, Cilley RE 1993; Physiology of extracorporeal life support. In: Arensman RM, Cornish JD (eds) Extracorporeal Life Support. Blackwell, Boston, 89–104.
Dudell G, Cornisch JD, Bartlett RH 1990; What constitutes adequate oxygenation? Pediatrics 85: 39–41.
van der Hoeven MAHBM, Maertzdorf WJ, Blanco CE 1995; Feasibility and accuracy of a fiberoptic catheter for the measurement of venous oxygen saturation in newborn infants. Acta Paediatr 84: 122–127.
van der Hoeven MAHBM, Maertzdorf WJ, Blanco CE 1996; Continuous central venous oxygen saturation (ScvO2) measurement using a fibreoptic catheter in newborn infants Arch Dis Child. 74:F177–F181.
Kreisberg RA 1980; Lactate homeostasis and lactic acidosis. Ann Intern Med 92: 227–237.
Mizock BA, Falk JL 1992; Lactic acidosis in critical illness. Crit Care Med 20: 80–93.
Sigma Chemical Company 1976; The quantitative determination of pyruvic acid and lactic acid in whole blood at 340 nm. Sigma Technical Bulletin No. 726-UV, 826-UV, St. Louis, MO, 1–21.
Brown EG, Krouskop RW, McDonnel FE, Monge CC, Winslow RE 1985; A technique to continuously measure arteriovenous oxygen content difference and P50 in vivo. J Appl Physiol 58: 1383–1389.
Mellits ED 1968; Statistical methods. In: Cheek DB (ed) Human Growth. Lea & Febiger, Philadelphia 19–38.
Fahey JT, Lister G 1987; Postnatal changes in critical cardiac output and oxygen transport in conscious lambs. Am J Physiol. 253:H100–H106.
Delivoria-Papadopoulos M, Martens RJ, Forster RE, Oski FA 1974; Postnatal changes in oxygen-hemoglobin affinity and erythrocyte 2,3-diphosphoglycerate in piglets. Pediatr Res 8: 64–66.
Mount LE, Ingram DL 1971; The Pig as a Laboratory Animal. Academic Press, London, 65–78.
Moss M, Moreau G, Lister G 1987; Oxygen transport and metabolism in the conscious lamb: the effects of hypoxemia. Pediatr Res 22: 177–183.
Schlichtig R, Cowden WL, Chaitman BR 1986; Tolerance of unusually low mixed venous oxygen saturation. Adaptations in the chronic low cardiac output syndrome. Am J Med 80: 813–818.
Reinhart K 1989; Monitoring O2 transport and tissue oxygenation in critically ill patients. In: Reinhart K, Eyrich K. (eds) Clinical Aspects of O2 Transport and Tissue Oxygenation. Springer Verlag, Berlin, 193–211.
Ceraianu AC, Nelson LD 1993; The significance of mixed venous oxygen saturation and technical aspects of continuous measurement. In: Edwards JD, Shoemaker WC, Vincent JL (eds) Oxygen Transport: Principles and Practice. Saunders, London, 99–124.
Astiz ME, Rackow EC, Kaufman B, Fay JL, Weil MH 1988; Relationship of oxygen delivery and mixed venous oxygen saturation in patients with sepsis and acute myocardial infarction. Crit Care Med 16: 655–658.
Rashkin MC, Bosken C, Baughman RF 1985; Oxygen delivery in critically ill patients. Relationship to blood lactate and survival. Chest 87: 580–584.
Danek SJ, Lynch JP, Weg JG, Dantzker DR 1980; The dependence of oxygen uptake on oxygen delivery in the adult respiratory distress syndrome. Am Rev Respir Dis 122: 387–395.
Welch E, Duara S, Suguihara C, Bandstra E, Bancalari E 1994; Validation of cardiac output measurement with non-invasive Doppler echocardiography by thermodilution and Fick methods in newborn piglets. Biol Neonate 66: 137–145.
Bizouarn P, Soulard D, Blanloeil Y, Guillet A, Goarin Y 1992; Oxygen consumption after cardiac surgery - a comparison between calculation by Fick's principle and measurement by indirect calorimetry. Intensive Care Med 18: 206–209.
Russell JA, Phang PT 1994; The oxygen delivery/consumption controversy. Approaches to management of the critically ill. Am J Respir Crit Care Med 149: 533–537.
Rubertson S, Grenvick Å, Wicklund L 1995; Blood flow and perfusion pressure during open-chest versus closed-chest cardiopulmonary resuscitation in pigs. Crit Care Med 23: 715–725.
Shiraishi H, Silverman NH, Rudolph AM 1993; Accuracy of right ventricular output estimated by Doppler echocardiograpy in the sheep fetus. Am J Obstet Gynecol 168: 947–953.
Mizock B 1989; Lactic Acidosis. Dis Mon 4: 235–300.
Wasserman K, Beaver WL, Whipp BJ 1990; Gas exchange theory and the acidosis (anaerobic) threshold. Circulation 81: suppl II 14–30.
Cain SM, Curtis SE 1991; Systemic and regional oxygen uptake and delivery and lactate flux in endotoxic dogs infused with dopexamine. Crit Care Med 19: 1552–1560.
Author information
Authors and Affiliations
Additional information
Supported by Abbott Inc., The Netherlands.
Rights and permissions
About this article
Cite this article
Van Der Hoeven, M., Maertzdorf, W. & Blanco, C. Mixed Venous Oxygen Saturation and Biochemical Parameters of Hypoxia during Progressive Hypoxemia in 10- to 14-Day-Old Piglets. Pediatr Res 42, 878–884 (1997). https://doi.org/10.1203/00006450-199712000-00026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199712000-00026
This article is cited by
-
CMR-based blood oximetry via multi-parametric estimation using multiple T2 measurements
Journal of Cardiovascular Magnetic Resonance (2016)