Abstract
Background
Increasing evidence recognizes the harm of excess oxygen to lungs, eyes, and brain of preterm infants, but not yet to the intestine. We assessed changes in splanchnic oxygenation during reoxygenation with 21% compared to 100% O2 in a newborn piglet model of perinatal asphyxia.
Methods
We randomized 25 piglets to control or intervention. Intervention groups underwent global hypoxia until acidosis and hypotension occurred. Piglets were reoxygenated for 30 min with 21% or 100% O2 and observed for 9 h. We continuously measured regional splanchnic oxygen saturation (rsSO2) using near-infrared spectroscopy (NIRS). We calculated mean rsSO2 and rsCoVar (as SD/mean). We measured PaO2 and SaO2, sampled from the right carotid artery.
Results
Reoxygenation after global hypoxia restored rsSO2. Reoxygenation with 100% O2 increased rsSO2 to values significantly higher than baseline. In intervention groups, rsCoVar decreased during observation compared to baseline. We found a correlation between rsSO2 and PaO2 (r = 0.420, P < 0.001) and between rsSO2 and SaO2 (r = 0.648, P < 0.001) in pooled data from the entire experiment.
Conclusion
Reoxygenation after global hypoxia improves splanchnic oxygenation, but is associated with reduced variability of rsSO2. Reoxygenation with 100% O2 exposes the intestine to hyperoxia. Splanchnic NIRS is able to detect intestinal hypoxia and hyperoxia.
Impact
-
Splanchnic oxygenation improves during reoxygenation after global hypoxia, though reoxygenation with 100% O2 exposes the intestine to hyperoxia.
-
Decreased variability of splanchnic oxygenation several hours after hypoxia and reoxygenation seems to be independent of the resuscitation strategy, and may indicate intestinal injury.
-
Splanchnic NIRS monitoring was able to detect intestinal hypoxia and exposure to hyperoxia, as evidenced by a strong correlation between splanchnic oxygenation and arterial oxygen content.
Similar content being viewed by others
Introduction
In preterm infants, the mechanisms that regulate intestinal oxygenation may be immature, leading to a higher susceptibility to changes in oxygen supply and oxygen demand compared to term infants.1 Therefore, preterm infants may frequently be exposed to intestinal hypoxia following changes from baseline conditions, such as enteral feeding, hypoxic hypoxemia, anemic hypoxemia, hypotension, and hypothermia.1,2 Intestinal hypoxia may compromise intestinal function and contribute to the development and progression of gastrointestinal diseases, including necrotizing enterocolitis (NEC).3 At the same time, increasing evidence recognizes the harmful effects of excess oxygen, which could trigger inflammation and oxidative stress.4,5 Therefore, the best resuscitation strategy for preterm infants in the delivery room remains unknown, although it seems a majority of very preterm infants require some amount of oxygen supplementation in the first 5 min to reach oxygen saturation targets.6 Nevertheless, preterm infants may be especially susceptible to oxidative stress due to immature antioxidant defenses.5,6 While oxygen toxicity has been documented for the lungs, eyes, and brain of preterm infants, the effects of hyperoxia on the intestine remain largely unknown.5
Monitoring of regional splanchnic oxygen saturation (rsSO2) using near-infrared spectroscopy (NIRS) has gained interest in the past decade and may be used to detect intestinal exposure to hypoxia and hyperoxia.7,8,9 NIRS is a non-invasive method that uses near-infrared light to measure the ratio of oxygenated to total hemoglobin (Hb) for the combined arterial, capillary, and venous sources in the underlying tissue.7,8,9 The vascular distribution is generally accepted to be approximately 20% arterial, 5% capillary, and 75% venous.8,9 Splanchnic NIRS monitoring was validated by correlating rsSO2 with blood flow velocity in the superior mesenteric artery (SMA) and with mixed venous oxygen saturation (SvO2), portal vein oxygen saturation (SpvO2), and umbilical vein oxygen (SuvO2) in both animal models and neonates.10,11,12,13,14 A recent study by Chen et al.14 demonstrated that splanchnic NIRS monitoring can accurately detect changes in splanchnic oxygenation in a piglet model of intestinal hypoxia produced by graded hypoxia or SMA ligation. The response of rsSO2 to different resuscitation strategies following global hypoxia has not previously been studied, but it has been documented that rsSO2 increases in response to red blood cell (RBC) transfusions.15,16,17,18,19,20,21 In addition, changes in variability of rsSO2 after RBC transfusion have been suggested to contribute to transfusion-associated NEC.16,20,22 Nevertheless, the mechanisms leading to intestinal injury following anemic hypoxemia and RBC transfusion may differ from those following hypoxic hypoxemia and reoxygenation.
The aims of this study were to assess the effects of global hypoxia and different resuscitation strategies on mean rsSO2 and variability of rsSO2 and to verify that splanchnic NIRS monitoring can accurately reflect changes in splanchnic oxygenation in a newborn piglet model of asphyxia.
Methods
Study design
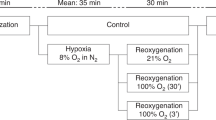
This study was part of a larger experiment that included 42 Noroc (LyxLD) piglets aged 6−36 h. We excluded piglets with hemoglobin (Hb) levels < 5.0 g/dL and signs of sickness. All piglets underwent anesthesia, ventilation, and surgical preparation according to previously described protocols in studies using this newborn piglet model of inflicted asphyxia.23,24 After surgery, piglets were stabilized during 1 h and randomized to a control group or an intervention group. Piglets in the intervention groups were subjected to global hypoxia, achieved by ventilation with 8% O2 in N2. We aimed to keep PaCO2 between 8.0 and 9.5 kPa by adding CO2 to mimic perinatal asphyxia. Hypoxia was continued until severe metabolic acidosis (arterial base excess < −20 mmol/L) or severe hypotension (mean arterial blood pressure < 20 mmHg) occurred. Piglets were then reoxygenated for 30 min according to randomization, i.e. 21% O2, 100% O2, or 100% O2 for 3 min followed by 21% O2 for 27 min. We will continue to refer to these intervention groups as “21% O2”, “100% O2 (30ʹ)”, and “100% O2 (3ʹ)”, respectively. The latter group was added, as a limited period of reoxygenation with 100% O2 may rapidly correct hypoxia, yet prevent prolonged exposure to hyperoxia. Piglets were observed for 9 h after reoxygenation. We chose this duration for observation to partially include the secondary energy failure that starts approximately 6 h after the initial incident.25 During this period of observation, piglets were ventilated with 21% O2. At the conclusion of the experiment, piglets were euthanized using an overdose of pentobarbital. Apart from hypoxia and reoxygenation, piglets in the control group underwent the same experimental setup. An overview of the study protocol is presented in Fig. 1. The experimental protocol has been approved by the Norwegian Council for Animal Research. Animals were handled in accordance with European Guidelines for Use of Experimental Animals, by researchers certified by the Federation of European Laboratory Animal Science Associations (FELASA).
Measurements
Throughout the study, we monitored heart rate, blood pressure, peripheral oxygen saturation (SpO2), and rectal temperature. We performed regular arterial blood gas analyses for temperature-corrected arterial acid−base status, arterial oxygen content, lactate, glucose, and Hb, sampled from the right carotid artery. In addition, we continuously monitored regional splanchnic oxygen saturation (rsSO2) with NIRS using an INVOS™ 5100C monitor (Medtronic, Dublin, Ireland) and a neonatal INVOS™ SomaSensor placed in the right lower quadrant of the abdomen, to target the terminal ileum and to avoid the bladder.14 We calculated mean rsSO2 and the coefficient of variation for rsSO2 (rsCoVar = SD/mean), as a measure of the variability of rsSO2. We used 5-min periods to pool and average rsCoVars.26
Statistical analyses
For analyses, we divided the study in four phases, which are referred to as “stabilization”, “hypoxia”, “reoxygenation”, and “observation” (Fig. 1). Given the long duration of observation, we analyzed this phase per hour. First, the normality of the data was assessed using the Kolmogorov−Smirnov test. Next, we assessed whether mean rsSO2 changed during the study compared to stabilization within groups using a Friedman repeated-measures analysis of variance by ranks test, with post hoc comparison to stabilization. To gain more insight into exposure to prolonged hypoxia and exposure to hyperoxia after the initial incident, we analyzed the reoxygenation phase in more detail. Then, we assessed whether mean rsSO2 differed between intervention groups compared to the control group throughout the study using a Kruskal−Wallis test, with post hoc comparison to control. We repeated these steps for rsCoVar. Finally, we assessed whether rsSO2 was associated with arterial oxygen pressure (PaO2) and arterial oxygen saturation (SaO2) using linear regression analysis for pooled data throughout the experiment.
Results
We successfully collected complete data sets in 25 out of 42 piglets (Fig. 2). We excluded 4 piglets that died, 2 piglets that reached a humane endpoint, and 14 that could not be completely evaluated because there were no NIRS monitors available. In three cases, there was a combination of these criteria. There were no differences in baseline characteristics among groups (Table 1).
The course of splanchnic oxygenation during hypoxia and reoxygenation
Mean rsSO2 was significantly lower during hypoxia compared to stabilization in the 21% O2 group (P = 0.014) (Fig. 3a–d). Mean rsSO2 was significantly higher during reoxygenation compared to stabilization in the 100% O2 (30ʹ) group (P = 0.002). We found that in piglets in the 100% O2 (30ʹ) and 100% O2 (3ʹ) groups, rsSO2 had returned to stabilization values at 3 min after the start of reoxygenation. In piglets in the 100% O2 (30ʹ) group, rsSO2 increased to values that were higher than during stabilization from 6 min after the start of reoxygenation onwards (P < 0.01). In contrast, in piglets in the 21% O2 group continued to have lower rsSO2-values at 3 (P = 0.001) and 6 min (P = 0.029) after the start of reoxygenation compared to stabilization (Fig. 4a–d). Mean rsSO2 was higher during observation compared to stabilization in the 100% O2 (30ʹ) and 100% O2 (3ʹ) groups (Fig. 3a–d). In the 100% O2 (30ʹ) group, mean rsSO2 was significantly higher during the eighth (P = 0.020) and the ninth hour (P = 0.013) of observation compared to stabilization. In the 100% O2 (3ʹ) group, mean rsSO2 was significantly higher during the fourth (P = 0.019), eighth (P = 0.014), and ninth hour (P = 0.004) of observation compared to stabilization.
During hypoxia, mean rsSO2 was significantly lower in all intervention groups compared to the control group (21% O2: P = 0.004, 100% O2 (30ʹ): P = 0.003, 100% O2 (3ʹ): P = 0.045). During reoxygenation, the mean rsSO2 at 3 min after the start of reoxygenation was lower compared to control in the 21% O2 group (P = 0.004). In the 100% O2 (30ʹ) group, the mean rsSO2 at 9 min after the start of reoxygenation was higher compared to control (P = 0.040). We did not find any differences in mean rsSO2 between control and intervention groups during stabilization and observation.
Variability of splanchnic oxygenation
We found lower variability of rsSO2 during observation compared to stabilization in all intervention groups (Fig. 5a–d). In the 21% O2 group, rsCoVar was significantly lower during the fourth (P = 0.045), fifth (P = 0.013), and eighth hour (P = 0.035) of observation compared to stabilization. In the 100% O2 (30ʹ) group, rsCoVar was significantly lower during the third to ninth hour of observation compared to stabilization (P < 0.05). In the 100% O2 (3ʹ) group, rsCoVar was significantly lower during the third (P = 0.039) and ninth hour (P = 0.030) of observation compared to stabilization.
During reoxygenation, rsCoVar was significantly higher in the 21% O2 group compared to the control group (P = 0.009). We did not find any differences in rsCoVar between control and intervention groups during stabilization, hypoxia, and observation.
Validation of splanchnic oxygenation monitoring
Finally, we assessed whether rsSO2 reflects changes in PaO2 and SaO2 (Fig. 6a, b). We found that mean rsSO2 was correlated with PaO2 (r = 0.420, P < 0.001) and SaO2 (r = 0.648, P < 0.001) in pooled data throughout the experiment. These correlations were significant in both control and intervention groups (data not shown). However, when the phases of the experiments were analyzed separately, we only found significant correlations between rsSO2 and PaO2 during hypoxia (r = 0.595, P < 0.001) and reoxygenation (r = 0.542, P < 0.001). Correlations between rsSO2 and SaO2 were significant during hypoxia (r = 0.481, P < 0.001), reoxygenation (r = 0.553, P < 0.001), and observation (r = 0.191, P = 0.016).
Discussion
In this study, using a newborn piglet model of perinatal asphyxia, we assessed the effects of global hypoxia and different resuscitation strategies on splanchnic oxygenation. We demonstrated that reoxygenation following global hypoxia improves splanchnic oxygenation, although reoxygenation with 100% O2 exposes the intestine to hyperoxia. In addition, we observed decreased variability of splanchnic oxygenation several hours after global hypoxia and subsequent reoxygenation, regardless of the applied resuscitation strategy. Finally, we found a strong association between splanchnic oxygenation and arterial oxygen content during hypoxia and reoxygenation.
To the best of our knowledge, we are the first to assess splanchnic oxygenation measured continuously and non-invasively with NIRS in a piglet model of asphyxia. Chen et al.14 have documented that rsSO2 decreases in a piglet model of graded whole-body hypoxia and SMA ligation, but they did not assess rsSO2 during reoxygenation or reperfusion. In pigs that underwent hemorrhage and fluid resuscitation, changes in gastric tissue oxygen saturation have been studied, using a probe placed through gastrotomy.27,28 In these studies, an increase in gastric tissue oxygen saturation was observed during resuscitation. Similarly, Mallick et al.29 found an increase in intestinal tissue oxygen saturation, measured on the surface of the intestine, during reperfusion after intestinal ischemia in rats. From these studies, we have learned that splanchnic oxygenation improves during resuscitation. Our results add to the existing literature that rsSO2 improves upon reoxygenation after global hypoxia and that reoxygenation with 100% O2 restores rsSO2 faster than reoxygenation with 21% O2, but also exposes the intestine to hyperoxia.
We found that variability of rsSO2 decreases several hours after global hypoxia and subsequent reoxygenation. Little is known about the physiological significance of variability of splanchnic oxygenation. We speculate that variability of rsSO2 indicates vascular adaptability to changes in oxygen supply and oxygen demand, similar to heart rate variability (HRV) that reflects changes in sympathetic and vagal activity.30,31 In preterm neonates, reduced HRV is associated with several pathologic conditions, including hypoxic-ischemic encephalopathy following perinatal asphyxia and NEC.30,31,32 Similarly, lower baseline variability of rsSO2 and reduced rsSO2-variability after RBC transfusion have been associated with NEC.20,33,34 Although the mechanisms leading to intestinal injury following anemia and RBC transfusion may differ from those following hypoxia and reoxygenation, we further speculate that reduced rsSO2-variability may be indicative of microvascular injury, leading to impaired microvascular blood flow and increased microvascular permeability as seen in intestinal ischemia-reperfusion.35 By definition, rsCoVar is dependent on mean rsSO2; thus, higher mean rsSO2 is accompanied by lower rsCoVar. Indeed, in the intervention groups, mean rsSO2 gradually increased during the observation period. However, rsCoVar decreased disproportionately, an effect that was most pronounced in the 21% O2 group. These results are in line with a recent meta-analysis on the use of high or low fraction of inspired oxygen in preterm infants in the delivery room.36 Although no significant differences in rates of NEC were demonstrated, there was a trend towards a higher rate of NEC in the low oxygen group. As we observed reduced variability of rsSO2 in all intervention groups, but not the control group, an intestinal microvascular injury may be a universal effect that occurs several hours after global hypoxia and reoxygenation, independent of the resuscitation strategy.
We verified that NIRS monitoring can accurately track splanchnic oxygenation during global hypoxia and reoxygenation by correlating rsSO2 with PaO2 and SaO2. Our results confirm the findings of previous studies that found an association between rsSO2 and arterial oxygen content.14,37 As we did not observe an association between rsSO2 and arterial oxygen content during stabilization and observation, we speculate that rsSO2 most accurately reflects changes in tissue oxygenation during substantial changes in arterial oxygen content. This is in line with previous findings by Brun et al.38 who demonstrated in newborn piglets that NIRS is less sensitive to cerebral tissue hypoxia during ischemic hypoxemia compared to hypoxic hypoxemia. This contrast may be explained by the contribution of arterial vascular sources to tissue oxygen saturation measured by NIRS. While the difference between arterial and venous saturation increases during ischemia, this difference decreases during hypoxia and hyperoxia.39,40 This may explain why we found correlations between rsSO2 and arterial oxygen content during hypoxia and reoxygenation, but not during stabilization and observation. Our results thus suggest that splanchnic NIRS monitoring can detect exposure to hypoxia and hyperoxia most accurately during substantial changes in arterial oxygen content.
We found that piglets were exposed to intestinal hyperoxia during reoxygenation with 100% O2 for 30 min, but not during reoxygenation with 100% O2 for 3 min. Although the effects of hyperoxia on the intestine remain largely unknown, previous studies have shown that higher fractions of inspired oxygen lead to a dose-dependent increase in urinary levels of oxidative stress biomarkers.41 Nevertheless, some amount of oxygen supplementation seems to be required, as we found continued exposure to intestinal hypoxia during the first 6 min after the start of reoxygenation with 21% O2. Additional research is needed to provide more insight into the effects of different fractions of inspired oxygen and durations of hypoxia on oxidative stress, inflammatory markers, and intestinal injury.
Our study has several strengths. We used a well-established piglet model of perinatal asphyxia. Given the age of 6−36 h of the piglets, our results best reflect postnatal hypoxia-reoxygenation. An important advantage of animal studies is the controlled, experimental setup that allows for a systematic approach to the research question. In addition, piglets share many physiologic similarities to human neonates, in particular regarding the gastrointestinal tract.42 Our study had a randomized setup that also included a control group, which allowed us to explore temporal changes within and between piglets. We also recognize some limitations. First, we had to exclude approximately 40% of the eligible piglets due to logistics, i.e. no NIRS monitor available. Only 7% of the eligible piglets were excluded because they were unable to overcome the extreme conditions that resulted from the inflicted asphyxia, i.e. they died or reached a humane endpoint. As a result, the sample size of our study was small. In addition, we chose not to correct for multiple testing, due to the exploratory nature of this study. Both may have increased the type II error rate. Second, we observed lower baseline Hb in the 100% O2 (3ʹ) group compared to control, although this did not reach statistical significance and did not seem to affect baseline rsSO2-values. Given the relative stability of Hb over time, this will not have affected changes in rsSO2, other than a potential underestimation of rsSO2 recovery or hyperoxia. Third, researchers who recorded the data were not blinded to the applied resuscitation strategy. Finally, we did not perform histological analyses of intestinal samples. Future studies will need to address whether the observed changes in mean rsSO2 and variability of rsSO2 correlate with intestinal injury.
In conclusion, splanchnic NIRS monitoring was able to detect intestinal hypoxia and exposure to hyperoxia in a piglet model of asphyxia. Global hypoxia and subsequent reoxygenation were associated with reduced variability of rsSO2, which may be an indication of intestinal injury.
References
Dotinga, B. et al. Maturation of intestinal oxygenation: a review of mechanisms and clinical implications for preterm neonates. Front. Pediatr. 8, 354 (2020).
Chaaban, H. & Stonestreet, B. Intestinal hemodynamics and oxygenation in the perinatal period. Semin. Perinatol. 36, 260–268 (2012).
Bazacliu, C. & Neu, J. Pathophysiology of necrotizing enterocolitis: an update. Curr. Pediatr. Rev. 15, 68–87 (2019).
Welsford, M. et al. Room air for initiating term newborn resuscitation: a systematic review with meta-analysis. Pediatrics 143, e20181825 (2019).
Saugstad, O. D., Oei, J. L., Lakshminrusimha, S. & Vento, M. Oxygen therapy of the newborn from molecular understanding to clinical practice. Pediatr. Res. 85, 20–29 (2019).
Welsford, M. et al. Initial oxygen use for preterm newborn resuscitation: a systematic review with meta-analysis. Pediatrics 143, e20181828 (2019).
Martini, S. & Corvaglia, L. Splanchnic NIRS monitoring in neonatal care: rationale, current applications and future perspectives. J. Perinatol. 38, 431–443 (2018).
Mintzer, J. & Moore, J. Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions. Pediatr. Res. 86, 296–304 (2019).
Seager, E., Longley, C., Aladangady, N. & Banerjee, J. Measurement of gut oxygenation in the neonatal population using near-infrared spectroscopy: a clinical tool? Arch. Dis. Child Fetal Neonatal Ed. 105, 76–86 (2020).
Kaufman, J., Almodovar, M. C., Zuk, J. & Friesen, R. H. Correlation of abdominal site near-infrared spectroscopy with gastric tonometry in infants following surgery for congenital heart disease. Pediatr. Crit. Care Med. 9, 62–68 (2008).
Gay, A. N. et al. Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J. Pediatr. Surg. 46, 1034–1040 (2011).
Gillam-Krakauer, M. et al. Correlation of abdominal rSO2 with superior mesenteric artery velocities in preterm infants. J. Perinatol. 33, 609–612 (2013).
Said, M., Niforatos, N. & Rais-Bahrami, K. Validation of near infrared spectroscopy to measure abdominal somatic tissue oxygen saturation in neonates. J. Neonatal Perinat. Med. 6, 23–30 (2013).
Chen, M. et al. Abdominal near-infrared spectroscopy in a piglet model of gastrointestinal hypoxia produced by graded hypoxia or superior mesenteric artery ligation. Pediatr. Res. 83, 1172–1181 (2018).
Bailey, S. M., Hendricks-Muoz, K. D., Wells, J. T. & Mally, P. Packed red blood cell transfusion increases regional cerebral and splanchnic tissue oxygen saturation in anemic symptomatic preterm infants. Am. J. Perinatol. 27, 445–453 (2010).
Marin, T. et al. Red blood cell transfusion-related necrotizing enterocolitis in very-low-birthweight infants: a near-infrared spectroscopy investigation. Transfusion 53, 2650–2658 (2013).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & Lagamma, E. F. Monitoring regional tissue oxygen extraction in neonates < 1250 g helps identify transfusion thresholds independent of hematocrit. J. Neonatal Perinat. Med. 7, 89–100 (2014).
Banerjee, J., Leung, T. S. & Aladangady, N. Effect of blood transfusion on intestinal blood flow and oxygenation in extremely preterm infants during first week of life. Transfusion 56, 808–815 (2016).
Banerjee, J., Leung, T. S. & Aladangady, N. Blood transfusion in preterm infants improves intestinal tissue oxygenation without alteration in blood flow. Vox Sang. 111, 399–408 (2016).
Kalteren, W. S. et al. Red blood cell transfusions affect intestinal and cerebral oxygenation differently in preterm infants with and without subsequent necrotizing enterocolitis. Am. J. Perinatol. 35, 1031–1037 (2018).
Aktas, S. et al. Effects of blood transfusion on regional tissue oxygenation in preterm newborns are dependent on the degree of anaemia. J. Paediatr. Child Health 55, 1209–1213 (2019).
Bailey, S. M., Hendricks-Muñoz, K. D. & Mally, P. V. Variability in splanchnic tissue oxygenation during preterm red blood cell transfusion given for symptomatic anaemia may reveal a potential mechanism of transfusion-related acute gut injury. Blood Transfus. 13, 429–434 (2015).
Benterud, T. et al. Perinatal asphyxia may influence the level of beta-amyloid (1-42) in cerebrospinal fluid: an experimental study on newborn pigs. PLoS ONE 10, e0140966 (2015).
Garberg, H. et al. Short-term effects of cannabidiol after global hypoxia-ischemia in newborn piglets. Pediatr. Res. 80, 710–718 (2016).
Hassell, K. J., Ezzati, M., Alonso-Alconada, D., Hausenloy, D. J. & Robertson, N. J. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch. Dis. Child Fetal Neonatal Ed. 100, F541–F552 (2015).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J. Neonatal Perinat. Med. 7, 199–206 (2014).
Cohn, S. M. et al. Splanchnic perfusion evaluation during hemorrhage and resuscitation with gastric near-Infrared spectroscopy. J. Trauma. 50, 629–634 (2001).
Varela, J. E. et al. Near-infrared spectroscopy reflects changes in mesenteric and systemic perfusion during abdominal compartment syndrome. Surgery 129, 363–370 (2001).
Mallick, I. H., Yang, W., Winslet, M. C. & Seifalian, A. M. Ischaemic preconditioning improves microvascular perfusion and oxygenation following reperfusion injury of the intestine. Br. J. Surg. 92, 1169–1176 (2005).
Javorka, K. et al. Heart rate variability in newborns. Physiol. Res. 66, S203–S214 (2017).
Bersani, I. et al. Heart rate variability as possible marker of brain damage in neonates with hypoxic ischemic encephalopathy: a systematic review. Eur. J. Pediatr. 180, 1335–45. (2021).
Doheny, K. K. et al. Diminished vagal tone is a predictive biomarker of necrotizing enterocolitis-risk in preterm infants. Neurogastroenterol. Motil. 26, 832–840 (2014).
Cortez, J. et al. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J. Matern Fetal Neonatal Med. 24, 574–582 (2011).
van der Heide, M., Hulscher J., Bos A. & Kooi, E. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr Res. 190, 148–155 (2021).
Turnage, R. H. et al. Splanchnic PGI2 release and “no flow” following intestinal reperfusion. J. Surg. Res. 58, 558–564 (2015).
Oei, J. L. et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: a meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 102, F24–F30 (2017).
Li, J. et al. Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near-infrared spectroscopy and direct measurements of systemic haemodynamic variables and oxygen transport after the Norwood procedure. Heart 92, 1678–1685 (2006).
Brun, N. C. et al. Near-infrared monitoring of cerebral tissue oxygen saturation and blood volume in newborn piglets. Am. J. Physiol. 273, H682–H686 (1997).
Leach, R. M. & Treacher, D. F. The relationship between oxygen delivery and consumption. Dis. Mon. 40, 301–368 (1994).
Perry, D. A. et al. Changes in tissue oxygen tension, venous saturation, and Fick-based assessments of cardiac output during hyperoxia. Acta Anaesthesiol. Scand. 63, 93–100 (2019).
Solberg, R., Andresen, J. H., Escrig, R., Vento, M. & Saugstad, O. D. Resuscitation of hypoxic newborn piglets with oxygen induces a dose-dependent increase in markers of oxidation. Pediatr. Res. 62, 559–563 (2007).
Douglas, W. R. Of pigs and men and research: a review of applications and analogies of the pig, sus scrofa, in human medical research. Sp. Life Sci. 3, 226–234 (1972).
Acknowledgements
This study was part of the research program of the Postgraduate School for Behavioural and Cognitive Neurosciences (BCN), University of Groningen, Groningen, The Netherlands. B.M.D. was financially supported by a Junior Scientific Master Class grant of the University of Groningen.
Author information
Authors and Affiliations
Contributions
B.M.D., E.M.W.K., R.S., and O.D.S. were involved in the design and execution of the study. All authors were involved in the final consensus process of the protocol and revised the manuscript critically for important intellectual content. B.M.D. drafted the manuscript and all other authors read, edited, and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dotinga, B.M., Solberg, R., Saugstad, O.D. et al. Splanchnic oxygen saturation during reoxygenation with 21% or 100% O2 in newborn piglets. Pediatr Res 92, 445–452 (2022). https://doi.org/10.1038/s41390-021-01819-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01819-6