Abstract
To investigate the role of high frequency oscillation (HFO) in promoting meconium clearance from the airway, we used a commercially available ventilator configured with maximal expiratory flow exceeding inspiratory flow(asymmetric HFO or AHFO). We hypothesized that AHFO would move meconium in an expiratory direction (toward the ventilator). We first tested our hypothesis in vitro and, later, in vivo using the neonatal piglet .In vitro experiments using a Plexiglas airway confirmed meconium movement in an expiratory direction when bias ratio was ≥2. For in vivo experiments, each piglet received a 3 mL/kg intratracheal bolus of a 44 g/100 mL meconium mixture followed by 45 min of mechanical ventilation. Then, in part 1, the piglet was placed in a 15 ° head down tilt position and randomized to either AHFO [ratio of inspiratory time/expiratory time (I:E) of 70:30] or HFO (I:E ratio of 30:70). After 30 min of either AHFO or HFO, the piglet was crossed over to the alternate strategy for an additional 30 min. For part 2, we maintained the piglet on either AHFO or HFO continuously for 4 h. Results demonstrate that, although there was a tendency for larger volumes of meconium to be aspirated from the airway during AHFO in part 1 experiments, there was no difference found in part 2. We also found no significant differences in blood gases or hemodynamic measurements between AHFO and HFO during the prolonged observation period in part 2 of our study. We conclude that AHFO is of no benefit in the treatment of meconium aspiration syndrome.
Similar content being viewed by others
Main
The mobilization and clearance of meconium is of vital importance in newborn infants with MAS. Complications from meconium aspiration include hypoxemia, pneumothorax, atelectasis, and chemical pneumonitis(1). When associated with pulmonary hypertension, mortality rates can approach 50%(2). Nasal and oropharyngeal suctioning, intratracheal suctioning, and chest physiotherapy are standard techniques for clearing meconium from the airway. Despite these measures, 5% of infants who are meconium-stained develop MAS and are at risk for life-threatening sequelae(1).
Although high frequency ventilation may be used clinically to treat selected infants with MAS(1), it has never before been considered a tool for enhancing meconium clearance. Nevertheless, previous animal studies have shown that, by adjusting ventilation so that expiratory flow exceeds inspiratory flow and inspiratory time exceeds expiratory time, mucus secretions can be mobilized toward the ventilator(3–5). When this technique is applied with an oscillatory ventilator, it is termed AHFO(3).
We hypothesized that AHFO would augment meconium clearance from the airway. We first studied the effects of AHFO and HFO on meconium transport in an airway model using a commercially available ventilator, the Sensormedics 3100 oscillator (Sensormedics Corp., Yorba Linda, CA). We noted the direction and linear displacement of meconium and measured the ratio of peak expiratory to peak inspiratory flow (bias ratio). To further elucidate the relationship of airflow to meconium movement, we examined the surface, physical, and transport properties of meconium. We then applied AHFO in an animal model of MAS using neonatal piglets. The amount of meconium-stained secretions suctioned from the proximal airway was quantified, and blood gases, systemic and pulmonary arterial pressures, and cardiac output were measured.
METHODS
First-pass meconium samples were collected over a 4-mo period from healthy full-term newborn infants born at Kapiolani Medical Center for Women and Children and Tripler Army Medical Center, Honolulu, HI. The samples were pooled, lyophilized, and reconstituted with distilled water to make a stock supply of meconium(6, 7). This preparation was further diluted with distilled water to make mixtures of 22 and 44 g/100 mL meconium. These concentrations were chosen to approximate the dilution of meconium with amniotic fluid in utero (22 g/100 mL, thin; 44 g/100 mL, thick meconium). Samples of the reconstituted undiluted meconium and each of the two dilutions were placed in sealed, airtight containers, frozen on dry ice, and sent by air freight to St. Louis for analysis of their surface, physical, and transport properties (cohesiveness, cough transportability,in vitro ciliary transportability, and hydrophilic wettability).
Experimental Protocol
In vitro study. The in vitro airway model consisted of a 60-cm long, 4.7-mm diameter (approximating the diameter of the term infant trachea) Plexiglas cylinder connected to the Sensormedics oscillator via a 3.5-mm diameter endotracheal tube. The Sensormedics was selected as the oscillator of choice because it was the only FDA approved high frequency oscillator available for clinical use at the time. Air flow was humidified and heated to 34 °C using a standard ventilator humidification system (Concha Thermal III Plus, Respiratory Care, Inc., Arlington Heights, IL)(Fig. 1). A sample of frozen meconium was warmed to 37°C, and a 0.05-mL volume of either the 22 or 44 g/100 mL meconium solution was injected into the lumen of the airway through a small hole in the cylinder. Before subjecting the sample to oscillatory flow, the hole was occluded with duoderm. Both AHFO and HFO were studied at frequencies of 5, 8, 10, and 15 Hz, amplitudes of 10, 20, 30, 40, 50, and 60 cm H2O, and at a constant Paw of 10 cm H2O. Linear displacement was measured(in cm) by the change in position of the leading edge of the meconium sample after two min of oscillatory ventilation. The direction of the sample movement(toward or away from the ventilator) was also noted. Peak air flow during AHFO and HFO was measured with a pneumotachometer (8300, Hans Rudolph Inc., Kansas City, MO), a differential pressure transducer (DP45-14, Validyne Engineering Corp., Northridge, CA), and a personal computer-based data acquisition system(AT-CODAS, Dataq Instruments, Inc., Akron, OH). Frequency response of the system was measured and found to be flat to a frequency of 60 Hz without changes in phase angle.
In vivo study. The study protocol was approved by the Institutional Animal Care and Use Committee at Tripler Army Medical Center. Investigators complied with the policies as prescribed in the U.S. Department of Agriculture Animal Welfare Act and the National Research Council's Guide for the Care and Use of Laboratory Animals. Facilities are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
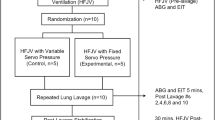
Part 1. Newborn piglets (6-7 d of age, n = 6) were anesthetized with pentobarbital (Wyeth Laboratories, Philadelphia, PA), intubated with a 4.0-mm diameter cuffed endotracheal tube, paralyzed with pancuronium (Astra Pharmaceutical Products Inc, Westborough, MA), and instrumented with femoral artery, peripheral venous, central venous, and pulmonary artery catheters as previously described(9). Baseline values were obtained on intermittent mandatory, conventional ventilation. Initial settings were fraction of inspired O2, 1.0; tidal volume, 10-15 mL/kg; and rate, 40 breaths/min. A 3 mL/kg bolus of 44 g/100 mL meconium was administered intratracheally over 10-15 s, and the peak inspiratory pressure was adjusted to maintain a constant tidal volume. Each piglet was then placed on conventional ventilation for 15 min to 1) stabilize the piglet after the bolus administration, 2) distribute the meconium throughout the airways, and 3) obtain baseline physiologic data. To establish oscillator settings and stabilize the animal on high frequency ventilation, the piglet was placed on HFO (I:E, 30:70) for 30 min with a pressure amplitude sufficient to achieve adequate chest wall movement (45-50 cm H2O), a Paw of 12 cm H2O, and a frequency of 8 Hz. Next, the animal was randomized to either AHFO or HFO, and placed in a 15° head-down tilt position. This was done so that gravity would augment the movement of meconium toward the oscillator, as described by Freitag et al.(3). We used a crossover study design where the same piglet was subjected to the alternate form of ventilation for 30 min intervals. Heart rate, systemic and pulmonary artery pressure, cardiac output, pulmonary artery wedge pressure, and blood gases were measured and/or calculated as in previous studies(9). Data were collected at the end of each 30-min period. Intratracheal suctioning was performed via an in-line suction catheter every 30 min or when large volumes of meconium were present and found to be obstructing the lumen of the endotracheal tube (no more than every 10 min). The suctioning technique involved advancing the catheter to the tip of the endotracheal tube and suctioning at -60 cm H2O for 10 s. The total volume of collected secretions was then measured and recorded. The total duration of the study(after randomization) was 2 h.
Part 2. An additional six piglets were prepared as in part 1. Fifteen minutes after meconium administration and conventional ventilation, the piglets were randomized to either HFO or AHFO and placed into a 15 ° head-down tilt position. The Paw was increased to 16 cm H2O and, instead of a crossover design, the animals were maintained on one strategy continuously for 4 h. The oscillator frequency was the same, but the amplitude was 10 cm H2O higher than used in part 1, which was necessary because of the difficulty in achieving adequate chest wall movement at the higher Paw. Blood gases, hemodynamic measurements, and endotracheal suctioning were all performed at 30-min intervals.
Lung Pathology
To examine the histopathologic consequences of meconium aspiration and the application of AHFO and HFO, lungs were fixed in 10% buffered formalin. Samples were taken from proximal and distal portions of all lobes. Sections were processed and embedded in paraffin. Slides were cut at 4 μm and stained with hematoxylin and eosin. Sections were examined by a single pathologist (J.L.K.) who was blinded to treatment protocols. Histologic findings were scored on a 4-point system modified from those previously described and considering current knowledge of the histologic progression of acute lung injury. Five features were evaluated including: inflation pattern, alveolar inflammatory reaction, alveolar hemorrhage, interstitial inflammatory reaction, and interstitial congestion(10). A total score of 5 denotes no significant change from normal, whereas a score of 20 represents severe injury in all five features.
Data Analysis
For the in vitro portion of our study, multiple linear regression was used to compare AHFO and HFO over the range of frequencies and pressure amplitudes used and to determine the parameters that correlated with the direction and linear displacement of meconium. Repeated measures two-way analysis of variance was used to analyze the impact of AHFO and HFO on blood gases and hemodynamic measurements. Finally, the t test was used to compare the total volume of meconium collected during each of the two ventilator strategies tested in our in vivo studies. A p value ≤0.05 was considered significant. All data are presented as the mean± SD.
RESULTS
In Vitro
With AHFO, meconium movement occurred in the expiratory direction at frequencies of 8, 10, and 15 Hz, whereas at the same frequencies with HFO, movement was in the inspiratory direction (Fig. 2). At a frequency of 5 Hz, however, movement occurred in the inspiratory direction with both HFO and AHFO. Meconium concentration did not affect linear displacement during AHFO (p = 0.06). Linear displacement was directly proportional to pressure amplitude at all frequencies (p< 0.0001). Frequency was also positively correlated with linear displacement, but only with the 22 g/100 mL mixture (p = 0.01). With AHFO, the ratio of peak expiratory to peak inspiratory flow (bias ratio) was 2.46 ± 0.11, 2.96 ± 0.18, and 2.31 ± 0.29 at frequencies of 8, 10, and 15 Hz, respectively. However, at these same frequencies, HFO generated bias ratios of 1.08 ± 0.07, 0.98 ± 0.11, and 1.28± 0.5, respectively. At a frequency of 5 Hz, there was no significant difference in the bias ratio during AHFO (1.54 ± 0.07) and HFO (1.49± 0.03).
Analysis of Physical, Transport, and Surface Properties of Meconium
These results are described in detail in previous work(8). The meconium samples were markedly abnormal compared with respiratory mucus. The ciliary transportability of meconium was lower than any respiratory secretion that we have studied. Undiluted meconium could not be cleared by a simulated maximal cough (that generated by a typical adult male). When diluted, meconium airflow clearability was low normal when compared with sputum cough clearability. The cohesiveness of undiluted meconium was slightly increased when compared with sputum and in the normal range when measured in either dilution. The diluted samples were able to wet the hydrophilic surface very well. The undiluted sample was too rigid to wet the hydrophilic surface. Both interfacial tension measured by platinum ring distraction and tenacity (work of distraction; the product of adhesiveness and cohesiveness) were extremely high; the undiluted meconium was the most tenacious body fluid that we have encountered. The 44 g/100 mL meconium was 25% more tenacious than the 22 g/100 mL mixture.
In Vivo
Part 1. Arterial Pco2 was higher (p = 0.0003) and pH lower (p < 0.0001) in piglets ventilated with AHFO compared with those with HFO. Pulmonary artery pressure was also higher during AHFO (p = 0.03). There were no differences in AHFO versus HFO for any of the other parameters measured (Table 1). The volume of meconium stained secretions aspirated tended to be greater during AHFO, but this value was not significant. These results are summarized in Figure 3A.
Part 2. With a higher Paw (16 versus 12 cm H2O in part 1), there were no differences in either blood gases or hemodynamic measurements in AHFO when compared with HFO(Table 2). There was also no significant difference in the volume of meconium-stained secretions suctioned, with a tendency for less during AHFO than during HFO (Fig. 3B).
Lung histopathology. Average scores for the sections of lung examined ranged from 7.5 to 12.5. The scores from the most severe areas of lung affected ranged from 9 to 13. There was no difference in the severity or type of lung injury between ventilation strategies (HFO or AHFO). A representative photomicrograph from a piglet treated with 4 h of HFO is shown in Figure 4. The total assigned score was 11; 1 for inflation pattern, 3 for alveolar inflammatory reaction, 1 for alveolar hemorrhage, 4 for interstitial inflammatory reaction, and 2 for interstitial congestion.
High power photomicrograph showing moderate lung injury after 4 h of HFO. Each feature was assigned a score from 1 (normal) to 5(severe). The total assigned score was 11; 1 for inflation pattern, 3 for alveolar inflammatory reaction, 1 for alveolar hemorrhage, 4 for interstitial inflammatory reaction, and 2 for interstitial congestion. No meconium was present in this section (hematoxylin and eosin, ×400).
DISCUSSION
To investigate the effects of AHFO on piglets with MAS, we developed both an in vitro system and an animal model. The results of our in vitro study are consistent with the observations of Chang et al.(11) and Kim et al.(12, 13) on the dynamics of two-phase gas liquid flow. These investigators characterized two-phase gas liquid flow(simultaneous flow of gas and liquid) in relation to clearance of secretions from the airway by delineating the necessary conditions for mucus clearance to occur; thickness of mucus layer relative to tube diameter, biased tidal breathing favoring expiratory flow, and the presence of shearing at the air-mucus interface. We measured peak air flows during HFO and AHFO, and were able to demonstrate that a bias ratio ≥2 consistently resulted in meconium movement in the expiratory direction. These findings are also in agreement with Chang et al.(11), except for the critical ratio at which the movement of mucus occurred in the expiratory direction (1.5 versus ≥2). The reduced transportability of meconium, which was demonstrated in this study, could have resulted in the requirement for a higher expiratory flow, and consequently, a greater bias ratio.
We were not surprised by the results of the analyses of the physical, transport, and surface properties of meconium. Meconium is known to be a viscous and tenacious substance consisting of gastrointestinal secretions, bile, bile acids, mucus, pancreatic juice, cellular debris, amniotic fluid, and swallowed vernix, lanugo, and blood(1). Our inability to duplicate the findings of Freitag et al.(3), who were able to demonstrate a beneficial effect of expiratory biased airway oscillation on artificial mucus clearance in intubated sheep, may in part be explained by the markedly reduced transportability of 44 g/100 mL meconium (7-10% that of human mucus). In Freitag et al.'s model there was also a constant flow of artificial mucus being infused intrabronchially. It is possible that our results would have been different with 22 g/100 mL meconium mixture, even though its transportability is only 29% that of mucus. We chose the 44 g/100 mL mixture with the belief that it would more closely approximate the clinical situation of “thick meconium.” It is also reasonable to consider why we had such disparate findings in the in vitro and animal studies. The physical characteristics of the lumen of a straight Plexiglas tube cannot be compared with the branching, compliant, mucus-lined living piglet lung with airways of varying diameter and an extensive surface area.
Our study was the first attempt to examine the effect of AHFO on meconium clearance. However, the relationship between HFO and mucociliary clearance has been previously addressed using a variety of experimental models. McEvoy et al.(14) demonstrated reduced mucociliary transport in dogs when exposed to high frequency low tidal volume ventilation. In contrast, George et al.(15) found improved mucociliary clearance when oral high frequency oscillation was superimposed on normal breathing in human adults. Finally, Freitag et al.(3) successfully applied the principles of two-phase gas liquid flow by demonstrating evidence of mucus clearance in sheep using AHFO.
It is important to emphasize the deterioration in the respiratory and hemodynamic status of the piglet during AHFO as observed in part 1 of our study, when we used a Paw of 12 cm H2O. In a previous study by Courtney et al.(16), using a rabbit model of surfactant depletion, no adverse effects were demonstrated during oscillatory ventilation with an inverse I:E ratio (1.5:1 and 2:1). The substantive differences in the pathophysiology of the meconium-injured lung and surfactant depletion secondary to repeated saline lung lavage may in part explain the lack of agreement in our findings. After part 1 of our in vivo study, we hypothesized that a higher Paw (16 versus 12 cm H2O) would improve the piglets' blood gas status, maintain airway patency, and improve clearance of meconium. We also speculated that we could further enhance meconium recovery with continuous exposure of the animal to AHFO. Our failure to show this may be explained by the higher Paw either moving the meconium more distally or dampening the effect of the pressure amplitude being delivered (despite the 10 cm H2O increase in amplitude), thereby reducing the shear force at the air-meconium interface. Alternatively, it is possible that at the lower MAP of 12 cm H2O the meconium remained more proximally within the airway, allowing for more successful recovery. We may have also limited the volume of meconium recovered by advancing our suction catheter only to the tip of the endotracheal tube(instead of more distally). Finally, the experimental model chosen to simulate meconium aspiration in the neonatal piglet may not accurately represent the clinical situation, thereby limiting the significance of our findings.
In conclusion, we were unable to demonstrate any benefit of AHFO on meconium clearance from the lung of the neonatal piglet. Due to this finding, as well as the marked deterioration in blood gas parameters seen with AHFO, it must be emphasized that this ventilation strategy cannot be recommended for use in human infants. Standard techniques of prevention and methods for clearing the airway after meconium aspiration should continue to be applied aggressively to prevent the serious and potentially life-threatening sequelae of MAS. Furthermore, we are encouraged by the study of Findlay et al.(17) on the effects of exogenous surfactant in the treatment of MAS and believe that surfactant treatment in conjunction with AHFO may be a promising area of future investigation.
Abbreviations
- HFO:
-
high frequency oscillation
- AHFO:
-
asymmetric high frequency oscillation
- I:E:
-
ratio of inspiratory time/expiratory time
- MAS:
-
meconium aspiration syndrome
- Paw:
-
mean airway pressure
References
Wiswell TE, Bent RC 1993 Meconium aspiration syndrome: unresolved issues. Pediatr Clin N Am 40: 955–981.
Morin FC, Stenmark KR 1995 Persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 151: 2010–2032.
Freitag L, Long WM, Kim CS, Wanner A 1989 Removal of excessive bronchial secretions by asymmetric high-frequency oscillations. J Appl Physiol 67: 614–619.
King M, Zidulka A, Phillips DM, Wight D, Gross D, Chang HK 1990 Tracheal mucus clearance in high-frequency oscillation: effect of peak flow rate bias. Eur Respir J 3: 6–13.
Benjamin RG, Chapman GA, Kim CS, Sackner MA 1989 Removal of bronchial secretions by two-phase gas liquid transport. Chest 95: 658–663.
Wiswell TE, Peabody SS, Davis JM, Slayter MV, Bent RC, Merritt TA 1994 Surfactant therapy and high-frequency jet ventilation in the management of a piglet model of the meconium aspiration syndrome. Pediatr Res 36: 494–500.
Bent R, Wiswell TE, Chang A 1992 Removing meconium from infant tracheae. What works best?. Am J Dis Child 146: 1085–1089.
Rubin BK, Tomkiewicz RP, Patrinos ME, Easa D 1996 The surface and transport properties of meconium and reconstituted meconium solutions. Pediatr Res 40: 834–838.
Easa D, Uyehara CFT, Stevens EL, Finn KC, Balaraman V, Sim H 1993 Pancuronium does not alter the hemodynamic status of piglets after normoxia or hypoxia. Pediatr Res 33: 365–372.
Davis JM, Dickerson B, Metlay L, Penney DP 1991 Differential effects of oxygen and barotrauma on lung injury in the neonatal piglet. Pediatr Pulmonol 10: 157–163.
Chang HK, Weber ME, King M 1988 Mucus transport by high-frequency nonsymmetrical oscillatory airflow. J Appl Physiol 65: 3: 1203–1209.
Kim CS, Rodriguez CR, Eldridge MA, Sackner MA 1986 Criteria for mucus transport in the airways by two-phase gas-liquid flow mechanism. J Appl Physiol 60: 901–907.
Kim CS, Iglesias AJ, Sackner MA 1987 Mucus clearance by two-phase gas-liquid flow mechanism: asymmetric periodic flow model. J Appl Physiol 62: 3:959-971
McEvoy RD, Davies NJH, Hedenstierna G, Hartman MT, Spragg RG, Wagner PD 1982 Lung mucociliary transport during high frequency ventilation. Am Rev Respir Dis 126: 452–456.
George RJD, Johnson MA, Pavia D, Agnew JE, Clarke SW, Geddes DM 1985 Increase in mucociliary clearance in normal man induced by oral high frequency oscillation. Thorax 40: 433–437.
Courtney SE, Weber KR, Spohn WA, Bender CV, Malin SW, Gotshall RW 1992 Cardiorespiratory effects of changing inspiratory to expiratory ratio during high-frequency oscillation in an animal model of respiratory failure. Pediatr Pulmonol 13: 113–116.
Findlay RD, Taeusch HW, Walther FJ 1996 Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 97: 48–52.
Acknowledgements
The authors thank the U.S. Army Health Services Command, Kapiolani Medical Center for Women and Children, and Sensormedics Corporation. We also thank the staff of the Hospital Laboratory and Dr. Jeffrey L. Killeen who performed the lung pathology studies, as well as the nursing staff who collected meconium. We acknowledge the technical expertise of Dr. Oscar Ramirez, Dr. Robert Tomkiewicz, Dr. Chikako Kishioka, and Titik Dian for analysis of meconium properties.
Author information
Authors and Affiliations
Additional information
Supported in part by the Research Centers in Minority Institutions Award, P20 RR/AI 11091, from the National Center for Research Resources, National Institutes of Health, and the Leahi Fund of the Hawaii Community Foundation.
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
Rights and permissions
About this article
Cite this article
Patrinos, M., Balaraman, V., Ku, T. et al. Promoting Meconium Clearance from the Lungs of the Neonatal Piglet with Asymmetric High Frequency Oscillation. Pediatr Res 42, 342–347 (1997). https://doi.org/10.1203/00006450-199709000-00015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199709000-00015