Abstract
We previously observed young lambs to be more tolerant of hypoxia; compared with older lambs, they accumulate lactate at a slower rate during comparable reduction in cardiac output, and have a greater percent decrease in cardiac output before onset of systemic lactate accumulation. To determine the mechanism of lactic acidosis and the cause for this “tolerance,” we reduced cardiac output progressively in seven chronically catheterized conscious lambs (16.4 + 5.1 d) and measured hepatic and gastrointestinal (GI) blood flow (radioactive microspheres) and delivery, uptake, and extraction of lactate and O2. Hepatic O2 consumption declined proportionately below a critical hepatic O2 delivery (≅2 mL O2/min/kg), corresponding to the systemic O2 delivery associated with the onset of systemic lactate accumulation. As hepatic O2 delivery decreased below the critical value, there was initially net hepatic lactate uptake and then a change to net production when the O2 delivery decreased below ≅1 mL O2/min kg. The GI tract had net lactate production at rest, but surprisingly switched to lactate uptake as cardiac output decreased. The mechanism of lactic acidosis was failure of hepatic lactate uptake to increase despite increased hepatic lactate delivery, as reported in adults subjects. However, in contrast, there was “true” hepatic dysfunction and lactate production only at the lowest levels of cardiac output, after onset of systemic lactate accumulation. Moreover, we speculate that tolerance of young lambs to hypoxia is at least due to two factors: 1) hepatic lactate uptake is maintained beyond the “critical” O2 delivery and fall in hepatic O2 consumption, and 2) there is a switch to lactate uptake by the GI tract serving to buffer the lactate.
Similar content being viewed by others
Main
When systemic O2 delivery is decreased by anemia, hypoxemia, or low cardiac output, arterial blood lactate concentration increases consistently when whole body O2 consumption starts to decrease, defining a“critical” level of O2 delivery(1–4). The cause for the increase in lactate concentration has been studied extensively in mature animals and has been attributed to dysfunctional hepatic metabolism(5–9). Berry(5) estimated that the capacity of the liver to take up lactate in the mature subject greatly exceeded the capacity of other tissues to produce it, and therefore any prolonged rise in blood lactate levels would occur only in the presence of hepatic dysfunction.
For a number of reasons it is not clear that these findings and conclusions by Berry are applicable to the immature subject. We have observed that the rate of accumulation of lactate during an hypoxic stress is less in the young lamb (<2 wk) than that in the mature subject under comparable conditions(10). The young lamb tolerates a greater proportional decrease in whole body oxygen transport before there is lactate accumulation(4). And, the neonatal liver has a much lower metabolic rate per mass (one-half adult values)(11, 12) and may be better tolerant of hypoxia. Accordingly, it is not clear whether the increase in arterial blood lactate during hypoxic conditions in the young lamb is caused by dysfunction of hepatic lactate metabolism. Rather hepatic function may be preserved whereas the lactate increase could be caused by decreased hepatic lactate delivery.
Therefore, in the present study we determined the mechanism for lactic acidosis in the young lamb during low cardiac output. We studied lambs ≤3 wk old because this group appears to be more resistant to hypoxia, based on reduced lactate accumulation(10). We adopted the methods of Gleason et al.(11) for directly sampling blood from the portal vein and hepatic vein in young lambs to measure hepatic O2 and lactate metabolism. These techniques allowed measurements of GI metabolism of O2 and lactate as well. We then limited O2 delivery by progressively lowering cardiac output and examined two potential mechanisms of altered lactate metabolism: 1) decreased hepatic lactate delivery, whereas hepatic lactate uptake remains normal; and 2) increased hepatic lactate delivery but failure of hepatic lactate uptake to increase in proportion to the excessive delivery.
METHODS
Subjects. The study was approved by the animal care and use committee at both Yale and the University of Pittsburgh. Seven healthy lambs of mixed breed were studied at approximately 2 wk of age.
Surgical preparation. The lamb was sedated with 10 mg/kg intramuscular ketamine; the lamb was shaved, and the skin was cleansed so that all surgery could be done with sterile technique. Using local lidocaine analgesia, vascular catheters were inserted through a skin incision and advanced from the hind leg to the abdominal aorta and pulmonary artery. The catheters were secured, and the skin was closed. An i.v. infusion of 5% dextrose, 0.9% NaCl solution was given at approximately 5 mL/kg/h and maintained throughout surgery and recovery. An endotracheal tube was inserted, and the lamb was ventilated with O2 and anesthetized with 1% halothane using a Harvard large animal ventilator.
A small midline abdominal incision was made, a section of the small intestine was exposed, and a polyvinyl catheter was inserted into a small mesenteric vein in one of the arcades and advanced so that the catheter tip was in the portal vein. The position of the catheter was verified at autopsy. The incision was closed, and the catheter was passed s.c. to the lamb's flank and brought to the skin.
Through a right thoracotomy in the seventh thoracic interspace, a purse-string suture was placed on the posterior inferior vena cava approximately 1 cm above the diaphragm. The inferior vena cava was punctured with a 19-gauge needle, and a polyvinyl catheter (outside diameter, 1.2 mm; inside diameter, 0.75 mm) was placed into the inferior vena cava through the purse string and advanced into the right or left hepatic vein. To ensure proper placement of the catheter in the hepatic vein, we compared lactate concentrations in blood sampled from the catheter with 1) that sampled by direct puncture of the inferior vena cava caudad to the insertion of the hepatic veins and 2) that in arterial blood. Hepatic venous blood always had the lowest lactate concentration, owing to hepatic lactate metabolism. Catheter position was also verified at autopsy. A chest tube was left in place in the pleural cavity for air and fluid drainage postoperative. The chest tube was removed the day after surgery. This catheter was also brought out s.c. to the lamb's flank.
Through a small neck incision, a tygon catheter (0.04 mm inside diameter) was inserted into the right carotid artery and advanced to the left ventricle: the pressure tracing was monitored to ensure proper placement. The jugular vein was then isolated, and a Foley catheter was inserted and advanced to the right atrium; again the pressure tracing was monitored to ensure proper placement. [We have previously described a method to lower cardiac output in conscious lambs by placing a Foley catheter into the right atrium; balloon inflation limits venous return and lowers cardiac output(13).]
All catheters were sutured in place, and the lamb was placed upright in a sling. To test adequacy of placement of the Foley catheter, the Foley balloon was inflated with warmed saline to determine the volume necessary to lower arterial pressure by approximately 50%. The balloon was then deflated, and all catheters were filled with heparinized saline solution (500 U/mL), capped, and fastened out of the reach of the animal. Each lamb was given intramuscular antibiotics (200 000 U of procaine penicillin G and 250 mg of dihydrostreptomycin sulfate) on the day of surgery and daily until the day of study.
The lambs were carefully observed during recovery from surgery and anesthesia. They were returned to their mothers when awake, vigorous, and able to walk. The lambs were housed with their mothers until the study. Each day they were weighed, and the catheters were flushed with heparin solution. They were studied 1 to 5 d postoperatively (see below).
Experimental protocol. The lamb fed freely from its mother until the time of study. At each study, the lamb was removed from its pen, weighed, and then placed in a sling so that it was supported in an upright position. The lamb was blindfolded in an attempt to keep it calm in the laboratory. The lamb then rested quietly in the sling for approximately 1 h while catheter patency was checked. The lambs did not feed during this time or at any time during the study. Based on previous studies in our laboratory(14), we assumed that all organs had baseline (fasting) blood flows by the time of the first regional blood flow determination.
The aortic and pulmonary arterial catheters were connected via sampling stopcocks to pressure transducers so that each pressure was monitored and recorded on a Beckman multichannel recorder (RG11). The lamb's snout was then secured in a mask to permit room air to be drawn in and expired gases to be collected for measurement of O2 consumption and CO2 production. All lambs were conscious and unsedated.
During the study, O2 consumption and CO2 production were monitored continuously. Data were collected only when the lamb had a stable blood pressure and constant O2 consumption and CO2 production for a period of at least three minutes. When this was obtained, systolic, diastolic, and mean pressures were recorded from the aorta and pulmonary artery at a rapid paper speed. Heart rate was measured from the aortic pressure trace. Blood samples (0.5-0.7 mL/sample) were slowly withdrawn from the aortic, pulmonary arterial, portal venous, and hepatic venous catheters for measurement of blood gas tensions, pH, O2 saturation, and lactate concentration. Hb and Hct were determined from the pulmonary arterial sample only. The blood removed was replaced with an equal volume of normal saline.(In previous studies with prolonged right atrial balloon inflation, we found that the Hb concentration tended to increase slightly with time. As we were attempting to lower cardiac output without changing Hb concentration or arterial O2 content, we elected to replace the blood drawn with normal saline and not blood.) Rectal and ambient temperatures were recorded. Immediately after the blood samples were obtained, radiolabeled microspheres were injected into the left ventricle to measure organ blood flow. Immediately before microsphere injection and for at least 45 s after completion of the injection, a reference sample was withdrawn from the descending aorta catheter at a rate of either 3.8 or 7.7 mL/min, depending upon the weight of the lamb.
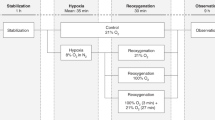
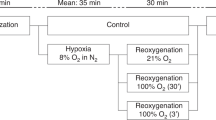
We obtained data at five different levels of cardiac output in each lamb (Fig. 1). As shown in a stylized schema in the figure, the relationship of whole body O2 consumption and systemic O2 transport has been shown to be biphasic for progressive low cardiac output in lambs(4, 13). Initially, O2 consumption remains relatively constant as systemic O2 transport decreases. Then, below a “critical” level of systemic O2 delivery (break point in the curve), the whole body O2 consumption declines in a nearly linear fashion with any further decreases in systemic O2 transport. Data were obtained at baseline (Fig. 1,no. 1). The right atrial balloon was then inflated progressively, in predetermined increments, to obtain data at two levels of low cardiac output above the critical level (Fig. 1,nos. 2 and 3) and then two further levels below the critical cardiac output (Fig. 1,nos. 4 and 5).
Schema of the study protocol. Data were obtained at five different levels of cardiac output and systemic O2 transport. Data were obtained at baseline (1), at two levels of lowered cardiac output (2 and 3) above the “critical” cardiac output (break point in the curve relating whole body O2 consumption to systemic O2 transport or cardiac output), and at two levels of lowered cardiac output (4 and 5) below the “critical” cardiac output.
Baseline was defined as the state in which all catheters were in place and the Foley balloon was fully deflated. After 20-30 min at baseline conditions, data were obtained (as above) and microspheres were injected. The Foley balloon was then inflated with warmed saline using a volume that was 20-30% of the balloon volume necessary to decrease blood pressure by one half(determined on the day of surgery). The animal was then allowed to adapt to the new cardiac output for at least 20 min, at which time a complete set of data determinations were made and microspheres injected. This usually took an additional 10 min, so that the lamb remained at each level of cardiac output for a total of 30 min. After microsphere injection, the balloon was inflated further. After the third microsphere injection (Fig. 1,no. 3), the balloon was inflated until O2 consumption fell by at least 20%, to be sure that we were below the critical cardiac output. After the fifth microsphere injection, the study was terminated, the balloon was deflated, and the animal was killed with an overdose of pentobarbital.
Measurements. Oxygen consumption and CO2 production were measured by a flow-through system in which ambient air was drawn at a constant rate through a face mask and was mixed with the expired gas from the lamb's nose and mouth. The fractional concentrations of O2 and CO2 were monitored continuously for calculation of O2 consumption and CO2 production as described previously(15). Blood gas tensions and pH were measured by standard electrodes at 37 °C (Radiometer BMS3MK2) and corrected to body temperature. Hbo2 was measured using a microoximeter (Radiometer OSM2). Hb (in g/dL) was measured by the cyanomethemoglobin method. Hct was determined by use of a microhematocrit centrifuge. Plasma lactate concentration (mmol/L) was measured with a rapid lactate analyzer (Yellow Spring Instrument, model 27).
Blood flow was determined by the microsphere method(16). Fifteen-micrometer microspheres labeled with either46 Sc, 51Cr, 85Sr, 95Nb, or 141Ce (DuPont NEN, Boston) were injected into the left ventricle while a reference blood sample was obtained from the descending aorta. At the end of a study, the lamb was killed, and the liver, spleen, stomach, small bowel, large bowel, and kidneys were removed. The organs were incinerated separately at 290 °C for 72 h and then pulverized and packed into counting vials. The amounts of each radionuclide in the organs and reference blood samples were measured with a well-type gamma scintillation counter (model 1185R, Tracer) and a multiple channel pulse height analyzer (Ultima II, Norland Corp., Chicago).
Hepatic arterial blood flow (Qha, in mL/min) was determined from the microspheres trapped in the liver. This assumes that an insignificant number of microspheres reach the liver via the portal circulation(17, 18). Portal venous blood flow (Qpv, in mL/min) was calculated as the sum of the arterial blood flows to the spleen, stomach, small bowel and large bowel. We have previously shown that the contribution of the pancreas and mesentery is trivial(14). [Although in very young lambs the ductus venosus may be patent and shunt the liver, the ductus venosus should be closed by 2 wk of age(19), and we assumed all portal venous blood supplied the liver.] The left and right kidney blood flows were determined separately to establish adequate mixing of the microspheres in the arterial circulation.
Calculations. Cardiac output (mL/min/kg) was calculated using the Fick equation, from the measured whole body O2 consumption and the arterial and mixed venous O2 contents. Systemic O2 transport (mL O2/min/kg) was calculated as the product of cardiac output and arterial O2 content.
Hepatic delivery of O2 (mL O2/min) and lactate (mmol/min) were calculated as the sum of the portal vein and hepatic artery contributions. Hepatic uptakes of O2 and lactate were calculated as the difference between the delivery rates and the rates of these substances exiting the liver in the hepatic vein. The formulas and assumptions for these calculations are included in the “Appendix.” Similar calculations for the GI tract are also included in the “Appendix.”
Statistical analysis. Data are reported as mean ± 1 SD unless otherwise noted. Changes in the portal contribution to total hepatic blood flow as cardiac output declined were detected by repeated measures analysis of variance. Statistical significance was assumed if p was less than 0.05. The relationship of hepatic blood flow and hepatic O2 delivery to the whole body O2 delivery was assessed by regression analysis using a least squares model.
RESULTS
The lambs had a mean age of 16.4 ± 5.1 d (range 10-22 d) with a mean body weight of 6.8 kg. They were studied 1-5 d postoperatively (mean 3.0 d) and were healthy at the time of study, as determined by weight gain and normal baseline values of arterial pH (7.46 ± 0.11), O2 saturation(95.1 ± 2.2%), arterial lactate concentration (0.82 ± 0.13 mmol/L), whole body O2 consumption (11.2 ± 1.3 mL O2/min/kg), and cardiac output (221 ± 54 mL/min/kg).
Baseline values of liver blood flow, O2, and lactate metabolism are included in Table 1. These data are comparable to those obtained in other laboratories(11, 20). The average liver weight was 185 ± 35 g. Liver blood flow was 29.3 ± 7.9% of the cardiac output. Portal venous flow provided 89.8 ± 6.7% of the total hepatic blood flow, with hepatic arterial blood flow supplying the remaining flow. Portal venous blood accounted for 85.2 ± 11.5% of the total hepatic O2 delivery. Liver O2 consumption constituted 20.0± 7.4% of the total O2 consumed by the lamb. There was consistently net uptake of lactate by the liver, which extracted 45 ± 17% of the lactate delivered to it.
Comparable baseline data for the GI tract (including stomach, small bowel, large bowel, and spleen) are presented in Table 2. The average total weight of these organs was 295.7 ± 50.7 g. GI blood flow represented 26.3 ± 7.1% of the cardiac output. Blood flow to the small bowel was the major component (≈56%) of the GI blood flow. GI O2 consumption accounted for 15.6 ± 5.0% of the whole body O2 consumption. The GI tract extracted approximately 33% of the O2 delivered to it under baseline conditions. We also calculated the net lactate uptake by the gut at these baseline conditions, and this value was usually negative, i.e. there was net production of lactate at baseline.
Combined data from all seven studies are shown in Figures 2–5. In Figure 2, whole body hemodynamic and metabolic data are plotted as a function of systemic O2 transport. Because Hb and arterial O2 saturation changed minimally with balloon inflation, arterial O2 content remained constant, systemic O2 transport is directly proportional to cardiac output (see equation in “Appendix” defining systemic O2 transport); thus, plots of the above variables versus cardiac output would be similar. With progressive decreases in systemic O2 transport and cardiac output, both whole body O2 consumption and arterial lactate concentration showed the same biphasic response as we have found previously. The “critical” level of O2 delivery for the combined data are at approximately 15.2 mL/min/kg [using the method of Samsel and Schumacker(21)]), representing approximately 60% of the baseline O2 transport. This result is similar to our prior results for lambs at this age(4). Mean arterial blood pressure was maintained with small decreases in cardiac output and O2 delivery, and then fell progressively. Mixed venous O2 saturation and content fell proportionately with decreasing systemic O2 transport, and the arterial-venous O2 content difference progressively widened. Owing to respiratory compensation in these spontaneously breathing lambs, arterial pH was maintained until the lowest levels of cardiac output (and highest lactate levels).
Portal and hepatic blood flow fell in direct proportion to the decrease in systemic O2 transport and cardiac output (Fig. 3); data points from individual studies are connected. Linear regression analysis of hepatic blood flow upon systemic O2 transport (y = 12.1x - 11.4) showed a good correlation (r = 0.86) which was highly significant (p < 0.001), and the 95% confidence intervals for the y intercept (-27.7, 5.0) included zero. Thus, hepatic blood flow remained a relatively constant fraction of cardiac output and systemic O2 transport. However, hepatic O2 delivery did not fall in direct proportion to systemic O2 transport and cardiac output. Linear regression analysis of hepatic O2 delivery upon systemic O2 transport (y = 0.97x - 3.7) showed a good correlation (r = 0.93; p < 0.001), but the line did not go through the origin (95% confidence limits of y intercept -4.7,-2.8). Thus, hepatic O2 delivery fell more rapidly than systemic O2 delivery, a finding which can be explained as follows. The majority of hepatic blood flow and O2 delivery (>86%) was derived from the portal vein, and this percentage did not change significantly as cardiac output declined. As blood flow to the GI tract decreased, the O2 content in the portal vein declined owing to increased GI O2 extraction; this caused hepatic O2 delivery to decrease out of proportion to systemic O2 transport. In every study, lactate delivery was increasing as systemic O2 transport declined below the critical level.
Hepatic metabolism of O2 and lactate are examined in Figure 4. All variables are now plotted as a function of hepatic O2 delivery. Again, data points from individual studies are connected. Hepatic O2 consumption displayed a biphasic relationship to declining O2 delivery, similar to what was observed for the whole body (Fig. 2); O2 consumption declined whenever O2 delivery was below 2 mL O2/min/kg, which defines an approximate critical level. The liver was able to increase O2 extraction to very high levels (as high as 0.9 or 90% extraction) in the face of severe limitations in O2 delivery. In spite of increasing lactate delivery to the liver, hepatic lactate uptake did not increase and, in fact, fell in all studies when hepatic O2 delivery was low. Furthermore, when hepatic O2 delivery fell below 1 mL/min/kg, the liver actually produced lactate, shown as a negative uptake (and negative extraction ratio).
GI metabolism of O2 and lactate are examined in a similar fashion in Figure 5. Variables are plotted as a function of net GI O2 delivery. As for the liver, the relationship between net GI O2 consumption and O2 delivery appears to be biphasic. The GI tract also was able to increase O2 extraction to very high levels as O2 delivery and blood flow progressively declined. The GI metabolism of lactate was, however, very different from that of the liver. As noted previously, there was a resting (baseline) net production of lactate by the GI organs, shown as a negative lactate uptake at high levels of O2 delivery. However, as GI O2 delivery declined below 3 or 4 mL O2/min kg, there was an increase in lactate extraction, resulting in a net uptake of lactate. Thus in all save one study, the GI tract was actually taking up lactate at the lowest levels of cardiac output and GI blood flow.
DISCUSSION
This study has delineated three issues concerning the onset of lactic acidosis with low cardiac output and the reported better tolerance of hypoxia in young subjects: 1) the lactic acidosis was related to a failure of hepatic lactate uptake to increase in proportion to the excessive lactate delivery, 2) there is hepatic “dysfunction” of lactate metabolism only when cardiac output is very low, and 3) there is delayed systemic lactate accumulation because of a switch from production to uptake of lactate by the GI tract, and an impairment of hepatic lactate metabolism that only occurs after hepatic O2 consumption declines substantially. These issues will be discussed in sequence.
The present studies were designed to differentiate two potential mechanisms of inadequate hepatic metabolism of lactate. The first possibility was that hepatic lactate delivery decreased as cardiac output declined. Although blood flow to the liver and GI tract was proportionately reduced with decreasing cardiac output, hepatic lactate delivery was maintained close to baseline levels and then increased to a variable degree as systemic O2 transport fell below approximately 15 mL O2/min/kg (Fig. 3). This corresponds to the level of systemic O2 transport at which arterial lactate increased abruptly (Fig. 2). Thus, hepatic lactate delivery did not decrease as hepatic blood flow declined, rather it stayed constant or increased. Our data support the second proposed mechanism that hepatic uptake of lactate does not keep pace with the increased lactate delivery. In fact, only one lamb increased lactate uptake at all. In six of seven studies hepatic lactate uptake stayed relatively constant as cardiac output decreased, and then rapidly fell as liver blood flow and O2 delivery declined further (Fig. 4).
To assess the onset of hepatic dysfunction we examined the relationship between hepatic O2 delivery and consumption, and lactate metabolism. As observed previously during hypoxemia(22), we found a biphasic relationship between hepatic O2 consumption and O2 delivery with low cardiac output (Fig. 4). Furthermore, lactate production only occurred after hepatic O2 consumption was decreased, and there was a range during which hepatic O2 consumption declined but there was still net lactate removal (Fig. 4). Systemic accumulation of lactate occurred before there was hepatic lactate production. Although hepatic lactate production added to the systemic accumulation of lactate at low levels of cardiac output, it is not a necessary condition for that accumulation to occur. There are very few other studies available which allow examination of the relationship of hepatic perfusion to O2 consumption and lactate metabolism(7, 8, 23), especially in the intact animal(6, 9). Schroder et al.(6) used cardiac tamponade to reduce cardiac output acutely in adult dogs. They too found that the liver only produced lactate when hepatic O2 consumption was decreased; however, they used a single low level of cardiac output and did not determine the conditions for transition from lactate uptake to production nor the relation of hepatic production to systemic accumulation. Schlichtig et al.(9) studied progressive decreases in cardiac output using hemorrhage in adult dogs and examined multiple markers of hepatic metabolism. They questioned whether the decrease in hepatic O2 consumption with low cardiac output represented 1) an“adaptive shutdown” in O2 demands to avoid hypoxia or 2) sustained O2 demands in excess of O2 supply indicating dysoxia. By evaluating hepatic mitochondrial redox state as a measure of O2 supply relative to demand, they found that the decrease in hepatic O2 consumption was not adaptive but represented tissue dysoxia coincident with the decrease in O2 consumption. They found an usual pattern of lactate metabolism that permitted neither determination of the relationship between hepatic O2 consumption and lactate metabolism nor the point at which hepatic lactate production occurred (there was net lactate production both at rest and with small decreases in cardiac output, net lactate uptake with moderate decreases in cardiac output, and net lactate production again at the lowest levels of cardiac output). Although the protocols for these studies in adult animals differed from ours, the finding of net lactate production when hepatic O2 consumption is decreased is similar to our data, and consistent with true hepatic dysfunction at very low cardiac output.
The exact mechanism of lactate uptake into hepatocytes is largely unknown, although two pathways have been identified, one which is carrier-mediated and saturable, where lactate is probably cotransported with hydrogen ion, and another which is nonsaturable and thought to represent simple diffusion(24–26). These authors concluded that under physiologic circumstances the carrier-mediated transport is more important; although the energetics of such a process are also uncertain, it appears most likely that lactate transport is energy-dependent, and therefore this process may be impaired during hypoxia as overall cellular energy stores are depleted. Alternatively, because the process is saturable, it is possible that the initial decreases in lactate extraction that we found may be due to saturation of the hepatic lactate transport system, especially with the smaller decreases in cardiac output and liver blood flow. Naylor et al.(27) have shown that, when lactate was infused into adult sheep with normal resting hemodynamics, hepatic lactate uptake is saturable at high (20 mmol/L) arterial lactate concentrations. No comparable data are available in young lambs, and our studies were not designed to evaluate hepatic saturation kinetics as we have only one portal lactate concentration at each level of cardiac output and hepatic blood flow. It is likely that the maximal hepatic lactate uptake (“Vmax”) will decrease as hepatic blood flow and O2 delivery are limited. Therefore, the hepatic lactate elimination mechanisms may be saturated at progressively lower portal lactate concentrations and, in our studies, coincident with progressively increasing levels of portal lactate. Accordingly, for moderate decreases in cardiac output and liver blood flow, we cannot differentiate whether the apparent impairment in hepatic lactate metabolism truly represents hepatic “dysfunction” or merely represents hepatic lactate saturation.
With more severe decreases in cardiac output and hepatic blood flow and O2 delivery, the liver was clearly dysfunctional in its handling of lactate. Not only did hepatic lactate uptake cease but there was net hepatic lactate production in six of seven lambs studied. A variety of factors have been reported to affect hepatic lactate uptake, including blood flow rate, hypoxia, acidosis, Pco2 of the perfusing medium, and catecholamines(7, 28, 29). In isolated perfused rat liver, Iles et al.(28) found that the combination of low flow plus acidosis impaired lactate uptake much more than low blood flow alone. Tashkin et al.(7) compared the effects of low hepatic blood flow and low Po2, and concluded that reducing the O2 tension of the perfusing blood caused greater changes in liver lactate metabolism than comparable reductions in hepatic O2 delivery by decreased flow. In our model of progressive low cardiac output, liver blood flow fell in proportion to the decline in cardiac output and hepatic O2 delivery decreased at an even faster rate (Fig. 3). This occurred because portal vein PO2 and O2 content decreased with enhanced GI O2 extraction (Fig. 5). This problem is compounded by the fact that portal blood flow in lambs represents 90% of the total hepatic blood flow, and the proportion did not change with changes in cardiac output (Fig. 3). Thus, with progressively reduced cardiac output, the liver was being perfused at progressively lower flow rates with progressively more hypoxemic (and acidotic) blood, establishing a“worst case scenario” for hepatic lactic metabolism. It is therefore not surprising to find liver hypoxia and dysfunction with associated lactate production at the lowest levels of cardiac output.
The response of the GI tract with regard to lactate uptake was just the opposite as that found for the liver. The GI tract progressed from net lactate production at rest to increasing net lactate uptake as GI blood flow and O2 delivery decreased. This result was surprising to us as we expected increasing lactate production as GI blood flow decreased. In the studies by Schroder et al.(6) of acute tamponade in dogs, mentioned above, the authors comment that in spite of the 67% decrease in cardiac output and portal blood flow during tamponade, the O2 consumption of the organs drained by the portal vein did not change and there was no net lactate production in this region. In our studies, GI O2 consumption was maintained over a wide range of O2 delivery and then declined. GI O2 extraction increased as O2 delivery decreased. We could find no other studies in young or adult animals investigating GI metabolism of lactate as a function of decreased blood flow. This pattern of net GI lactate production changing to net uptake has been described in adult sheep during steady state infusions of lactate into the inferior vena cava(27). The mechanism of this uptake and the fate of the lactate taken up by the GI cells are unknown. Because we sampled only from the portal vein, we do not know the contribution to lactate uptake of the individual organs drained by the portal vein. However, GI lactate uptake does serve to lessen the lactate delivery to the liver and help delay the onset of systemic lactate accumulation. Whether this process is unique to young lambs or persists in adult sheep is not known but it may help explain the differential lactate accumulation rates we have previously reported between young and older lambs for comparable decreases in cardiac output(10).
In addition, the liver of the young lamb appeared fairly tolerant of decreased blood flow as the onset of hepatic lactate production did not coincide with the fall in hepatic O2 consumption, but occurred only when O2 consumption had declined substantially. It has been proposed that there are a variety of synthetic and maintenance cellular processes that are not essential for survival and may be shut off at times of stress, so-called “nonessential metabolism”(30–32). This alteration in metabolic demand at times of limited O2 supply corresponds to “O2 conformity” as described by Hochachka and Guppy(33). If hepatic metabolic demands can be acutely lowered when cardiac output and hepatic blood flow are reduced, the matching of O2 supply to demand would be improved and hepatic hypoxia delayed. We could not determine whether part of the decrease in hepatic O2 consumption represents loss of nonessential metabolism and therefore delayed the onset of lactate production.
It is not clear whether our findings in the young lamb can be generalized to mature sheep. All of our lambs were 22 d or less at the time of the study and nonruminant, because they were housed their mothers and nursed until study. We have not been able to find reports of hepatic lactate uptake by adult sheep. We would speculate that older sheep would have a greater ability to take up and metabolize lactate, as the adult ruminant must supply all of its glucose needs by gluconeogenesis. Clark et al.(34) showed that, in isolated lamb hepatocytes, the gluconeogenic rate from lactate increased commensurate with rumen activity, with the transition from nonruminant to ruminant digestion occurring at about 30 d of age. Thus, the older lamb may actually be more efficient at metabolizing lactate. However, the hepatic O2 consumption of the mature sheep is considerably higher (almost double) compared with the newborn lamb and may be, therefore, more susceptible to hypoxia when O2 supply is limited(11, 12). Mature sheep also have resting net lactate production by the GI tract(35), as did our younger lambs. The response of the mature GI tract to progressive low cardiac output is unknown. Whether the mature GI tract will increase lactate uptake and help to buffer lactate accumulation and hepatic lactate delivery has not been studied.
To summarize, we found that, in the face of progressively decreasing cardiac output and liver blood flow, hepatic lactate delivery is maintained or increased, whereas hepatic lactate uptake and extraction are impaired. At very low cardiac output, consequent to hypoxia the liver produces lactate and contributes to systemic lactic acidosis. Surprisingly, the GI tract had progressively increasing lactate uptake as its blood flow and O2 delivery were decreasing, thereby delaying the onset of systemic accumulation. At moderate decreases in cardiac output, preservation of hepatic lactate metabolism and the uptake by the GI tract may contribute to the apparent tolerance of the young subject to global hypoxia.
Abbreviations
- GI:
-
gastrointestinal
- Hct:
-
hematocrit
- Hbo2:
-
fractional Hb oxygen saturation
References
Cain SM 1977 Oxygen delivery and uptake in dogs during anemic and hypoxic hypoxia. J Appl Physiol 42: 228–234.
Simmons DH, Alpas AP, Tashkin DP, Coulson A 1978 Hyperlactatemia due to arterial hypoxemia or reduced cardiac output, or both. J Appl Physiol 45: 195–202.
Moss M, Moreau G, Lister G 1987 Oxygen transport and metabolism in the conscious lamb: the effects of hypoxemia. Pediatr Res 22: 177–183.
Fahey JT, Lister G 1987 Postnatal changes in critical cardiac output and O2 transport in conscious lambs. Am J Physiol 253:H100–H106.
Berry MN 1967 The liver and lactic acidosis. Proc R Soc Med 60: 52–54.
Schroder R, Gumpert JRW, Pluth JR, Elthringham WK, Jenny ME, Zollinger RM 1969 The role of the liver in the development of lactic acidosis in low flow states. Postgrad Med J 45: 566–570.
Tashkin DP, Goldstein PJ, Simmons DH 1972 Hepatic lactate uptake during decreased liver perfusion and hypoxemia. Am J Physiol 223: 968–974.
Samsel RW, Cherqui D, Pietrabissa A, Sanders WM, Roncella M, Emond JC, Schumacker PT 1991 Hepatic oxygen and lactate extraction during stagnant hypoxia. J Appl Physiol 70: 186–193.
Schlichtig R, Klions HA, Kramer DJ, Nemoto EM 1992 Hepatic dysoxia commences during O2 supply dependence. J Appl Physiol 72: 1499–1505.
Fahey JT, Lister G 1989 Response to low cardiac output: developmental differences in metabolism during oxygen deficit and recovery in lambs. Pediatr Res 26: 180–187.
Gleason CA, Roman C, Rudolph AM 1985 Hepatic O2 consumption, lactate uptake, and glucose production in neonatal lambs. Pediatr Res 19: 1235–1239.
Harrison F, Linzell J, Paterson J 1972 Oxygen consumption by the liver of the conscious sheep. J Anat 111: 330
Fahey JT, Lister G 1985 A simple method for reducing cardiac output in the conscious lamb. Am J Physiol 249:H188–H192.
Edelstone DI, Holzman IR 1981 Gastrointestinal tract O2 uptake and regional blood flows during digestion in conscious newborn lambs. Am J Physiol 241: G289–G293.
Lister G, Hoffman JIE, Rudolph AM 1974 Oxygen uptake in infants and children: a simple method of measurement. Pediatrics 53: 656–662.
Heymann MA, Payne BD, Hoffman JIE, Rudolph AM 1977 Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis 20: 55–79.
Delaney JP 1969 Arteriovenous anastomotic blood flow in the mesenteric organs. Am J Physiol 216: 1556–1561.
Greenway CV, Murthy VS 1972 Effects of vasopressin and isoprenaline infusions on the distribution of blood flow in the intestine; criteria for the validity of microsphere studies. Br J Pharmacol 46: 177–188.
Botti JJ, Edelstone DI, Caritis SN, Mueler-Heubach E 1982 Portal venous blood flow distribution to liver and ductus venosus in newborn lambs. Am J Obstet Gynecol 144: 303–308.
Edelstone DI, Holzman IR 1981 Oxygen consumption by the gastrointestinal tract and liver in conscious new born lambs. Am J Physiol 240:G297–G304.
Samsel RW, Schumacker PT 1988 Determination of the critical O2 delivery from experimental data: sensitivity to error. J Appl Physiol 64: 2074–2082.
Edelstone DI, Paulone ME, Holzman IR 1984 Hepatic oxygenation during arterial hypoxemia in neonatal lambs. Am J Obstet Gynecol 150: 513–518.
Lutz J, Henrich H, Bauereisen E 1975 Oxygen supply and uptake in the liver and the intestine. Pflugers Arch 360: 7–15.
Metcalfe HK, Monson JP, Welch SG, Cohen RD 1986 Inhibition of lactate removal by ketone bodies in rat liver. J Clin Invest 78: 743–747.
Monson JP, Smith JA, Cohen RD, Iles RA 1982 Evidence for a lactate transporter in the plasma membrane of the rat hepatocyte. Clin Sci 62: 411–420.
Fafournoux P, Demigne C, Christian R 1985 Carrier-medicated uptake of lactate in rat hepatocytes. J Biol Chem 260: 292–299.
Naylor JM, Kronfeld DS, Freeman DE, Richardson D 1984 Hepatic and extrahepatic lactate metabolism in sheep: effects of lactate loading and pH. Am J Physiol 247: E747–E755.
Iles RA, Cohen RD, Baron PG 1981 The effect of combined ischaemia and acidosis on lactate uptake and gluconeogenesis in the perfused rat liver. Clin Sci 60: 537–542.
Iles RA, Cohen RD, Baron PG, Smith JA, Henderson RM 1981 The effect of adrenaline on hepatic lactate uptake in the acidotic partially ischaemic rat liver. Clin Sci 60: 543–548.
Sidi D, Kuipers JR, Teitel D, Heymann MA, Rudolph AM 1983 Developmental changes in oxygenation and circulatory responses to hypoxemia in lambs. Am J Physiol 245:H674–H682.
Robin E 1978 Overview, dysoxia-abnormalities of tissue oxygen use. In: Lenfant C (ed) Extrapulmonary Manifestations of Respiratory Disease. Marcel Dekker, New York, pp 3–12.
Adams RP, Cain SM 1983 Total and hindlimb oxygen deficit and “repayment” in hypoxic anesthetized dogs. J Appl Physiol 55: 913–922.
Hochachka P, Guppy M 1987 Metabolic Arrest and the Control of Biological Time. Harvard University Press, Cambridge, MA, pp 10–35.
Clark M, Filsell O, Jarrett I 1976 Gluconeogenesis in isolated intact lamb liver cells. Biochem J 156: 671–680.
Armentano L 1992 Ruminant hepatic metabolism of volatile fatty acids, lactate and pyruvate. J Nutr 122: 838–842.
Acknowledgements
The authors thank Andrea O. Ray, Anthony Battelli, and Vincent Santucci for their expert technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by a Child Health Research Grant from the Charles H. Hood Foundation. D.J.S. was supported by a National Heart, Lung, and Blood Institute, Training Grant HL07272. D.I.E. was supported by National Institutes of Health Grant HD19092. G.L. is an Established Investigator of the American Heart Association.
APPENDIX
APPENDIX
Calculations. Oxygen contents. Aortic (a), pulmonary arterial (mixed venous, v), portal venous (pv), and hepatic venous (hv) blood O2 contents (Cao2, Cvo2, Cpvo2, Chvo2, in mL O2/dL) were calculated from the respective Po2 (mm Hg), Hbo2 (fractional), and Hb (g/dL) as: Equation where 0.003 (mL O2/dL plasma mm Hg) is the Bunsen solubility coefficient for O2 in plasma at 37 °C. x = a, v, pv, hv.

Cardiac output. Qt, in mL/min/kg, was calculated using the Fick equation, from whole body O2 consumption (Vo2 in mL/min kg), Cao2, and Cvo2 as: Equation

Systemic O2 transport. Systemic O2 transport(SOT, in mL O2/min/kg) was calculated from its definition:Equation

Hepatic O2 delivery. Oxygen delivered to the liver (mL O2/min) was calculated as the sum of portal vein and hepatic artery contributions, with blood flows (Qpv, Qha, Qh) in mL/min:Equation

Hepatic O2 consumption. Oxygen consumption by the liver (mL O2/min) was calculated by: Equation where Qh = total liver blood flow = Qpv + Qha.

Hepatic O2 extraction. Hepatic O2 extraction was calculated as the quotient of hepatic O2 consumption and hepatic O2 delivery.
Hepatic lactate delivery. The lactate delivered to the liver(mmol/min) was calculated as for O2 delivery, substituting [L] (plasma lactate concentration, in mmol/L plasma) for O2 content.Equation where PCV = packed cell volume = Hct/100; this assumes that packed cell volume is the same in portal and arterial vessels.

Net hepatic lactate uptake (mmol/min).Equation This equation yields uptake of lactate from plasma and assumes that this is similar to uptake from blood because erythrocyte lactate equilibrates slowly with plasma(27).

Actual hepatic lactate extraction ratio.Equation We noted that the hepatic artery flows are a small percentage of the total liver blood flow (<10%) and the majority of hepatic blood flow at all levels of cardiac output was predominately portal flow (i.e. Qha ≪ Qpv; Qh ≅ Qpv). The formula for hepatic lactate extraction is greatly simplified if Qha is assumed to be zero and Qpv= Qh. Making these substitutions yields:

Estimated hepatic lactate extraction ratio.Equation As seen, all flow terms cancel and the estimation requires only measurement of lactate in the portal and hepatic veins. To examine the validity of this assumption, the estimated hepatic lactate extraction is plotted as a function of the actual hepatic lactate extraction in Figure 6. As seen, the linear regression analysis shows an excellent correlation (r = 0.99) with a slope close to one (0.99) and a SEM of the estimate of 0.036. The estimated lactate extraction is obviously a much easier measure to make, because it requires no measurement of blood flow, but only the ability to sample from the hepatic vein and portal vein. This estimation is accurate in the lamb because portal blood flow accounts for 90% of the total hepatic blood flow. The estimation may be less valid in species who have a larger proportion of the hepatic blood flow supplied by the hepatic artery, for example in man or pigs where the hepatic artery supplies approximately one-third of the hepatic blood flow. In the lamb, this estimation can be useful for assessing lactate extraction during times or studies where flow measurements are not possible or are limited in number.

Net GI O2 consumption.Equation where GI includes stomach, small bowel, large bowel, and spleen.

Net GI lactate uptake. Equation

GI lactate extraction. Equation

Rights and permissions
About this article
Cite this article
Fahey, J., Lister, G., Sanfilippo, D. et al. Hepatic and Gastrointestinal Oxygen and Lactate Metabolism during Low Cardiac Output in Lambs. Pediatr Res 41, 842–851 (1997). https://doi.org/10.1203/00006450-199706000-00008
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199706000-00008