Abstract
Background
In preterm infants, intestinal hypoxia may partly contribute to the pathophysiology of necrotizing enterocolitis through changes in gene expression. Splanchnic hypoxia can be detected with monitoring of regional splanchnic oxygen saturation (rsSO2). Using a piglet model of asphyxia, we aimed to correlate changes in rsSO2 to gene expression.
Methods
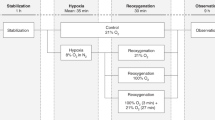
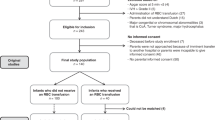
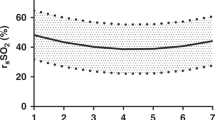
Forty-two newborn piglets were randomized to control or intervention groups. Intervention groups were subjected to hypoxia until they were acidotic and hypotensive. Next, they were reoxygenated for 30 min according to randomization, i.e., 21% O2, 100% O2, or 100% O2 for 3 min followed by 21% O2, and observed for 9 h. We continuously measured rsSO2 and calculated mean rsSO2 and variability of rsSO2 (rsCoVar = SD/mean). Samples of terminal ileum were analyzed for mRNA expression of selected genes related to inflammation, erythropoiesis, fatty acid metabolism, and apoptosis.
Results
The expression of selected genes was not significantly different between control and intervention groups. No associations between mean rsSO2 and gene expression were observed. However, lower rsCoVar was associated with the upregulation of apoptotic genes and the downregulation of inflammatory genes (P < 0.05).
Conclusion
Our study suggests that hypoxia and reoxygenation cause reduced vascular adaptability, which seems to be associated with the upregulation of apoptosis and downregulation of inflammation.
Impact
-
Our results provide important insight into the (patho)physiological significance of changes in the variability of rsSO2. Our findings may advance future research and clinical practice regarding resuscitation strategies of preterm infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2019).
Neu, J. & Walker, W. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Dotinga, B. et al. Maturation of intestinal oxygenation: a review of mechanisms and clinical implications for preterm neonates. Front. Pediatr. 8, 354 (2020).
Du, J. et al. Irisin pretreatment ameliorates intestinal ischemia/reperfusion injury in mice through activation of the Nrf2 pathway. Int. Immunopharmacol. 73, 225–235 (2019).
Karna, P., Senagore, A. & Chou, C. Comparison of the effect of asphyxia, hypoxia, and acidosis on intestinal blood flow and O2 uptake in newborn piglets. Pediatr. Res. 20, 929–932 (1986).
Haase, E. et al. Resuscitation with 100% oxygen causes intestinal glutathione oxidation and reoxygenation injury in asphyxiated newborn piglets. Ann. Surg. 240, 364–373 (2004).
Johnson, S. et al. Effects of N-acetylcysteine on intestinal reoxygenation injury in hypoxic newborn piglets resuscitated with 100% oxygen. Neonatology 96, 162–170 (2009).
Luo, H., Guo, P. & Zhou, Q. Role of TLR4/NF-κB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS One 7, e46291 (2012).
Tchessalova, D. & Tronson, N. C. Enduring and sex-specific changes in hippocampal gene expression after a subchronic immune challenge. Neuroscience 428, 76–89 (2020).
Weger, B. D. et al. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab. 29, 362–382.e8 (2019).
Okyar, A. et al. Sex-, feeding-, and circadian time-dependency of P-glycoprotein expression and activity – implications for mechanistic pharmacokinetics modeling. Sci. Rep. 9, 1–15 (2019).
Chen, M. et al. Abdominal near-infrared spectroscopy in a piglet model of gastrointestinal hypoxia produced by graded hypoxia or superior mesenteric artery ligation. Pediatr. Res. 83, 1172–1181 (2018).
Mintzer, J. P. & Moore, J. E. Regional tissue oxygenation monitoring in the neonatal intensive care unit: evidence for clinical strategies and future directions. Pediatr. Res. 86, 296–304 (2019).
Kalteren, W. S. et al. Red blood cell transfusions affect intestinal and cerebral oxygenation differently in preterm infants with and without subsequent necrotizing enterocolitis. Am. J. Perinatol. 35, 1031–1037 (2018).
van der Heide, M., Hulscher, J., Bos, A. & Kooi, E. Near-infrared spectroscopy as a diagnostic tool for necrotizing enterocolitis in preterm infants. Pediatr. Res. 90, 148–155 (2021).
Kalteren, W. S., Bos, A. F., van Oeveren, W., Hulscher, J. B. F. & Kooi, E. M. W. Neonatal anemia relates to intestinal injury in preterm infants. Pediatr. Res. 91, 1452–1458 (2022).
Benterud, T. et al. Perinatal asphyxia may influence the level of beta-amyloid (1-42) in cerebrospinal fluid: an experimental study on newborn pigs. PLoS One 10, e0140966 (2015).
Garberg, H. et al. Short-term effects of cannabidiol after global hypoxia-ischemia in newborn piglets. Pediatr. Res. 80, 710–718 (2016).
Hassell, K. J., Ezzati, M., Alonso-Alconada, D., Hausenloy, D. J. & Robertson, N. J. New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch. Dis. Child Fetal Neonatal Ed. 100, F541–F552 (2015).
Solberg, R. et al. Resuscitation of hypoxic newborn piglets with supplementary oxygen induces dose-dependent increase in matrix metalloproteinase-activity and down-regulates vital genes. Pediatr. Res. 67, 250–256 (2010).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J. Neonatal Perinat. Med. 7, 199–206 (2014).
Zmora, O. et al. Prophylactic antenatal N-Acetyl Cysteine administration combined with postnatal administration can decrease mortality and injury markers associated with necrotizing enterocolitis in a rat model. PLoS One 15, e0233612 (2020).
Singhal, R. & Shah, Y. M. Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 295, 10493–10505 (2020).
Gribar, S. C. et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 182, 636–646 (2009).
Chan, K. Y. Y. et al. Genome-wide expression profiles of necrotizing enterocolitis versus spontaneous intestinal perforation in human intestinal tissues: dysregulation of functional pathways. Ann. Surg. 260, 1128–1137 (2014).
Bazacliu, C. & Neu, J. Pathophysiology of necrotizing enterocolitis: an update. Curr. Pediatr. Rev. 15, 68–87 (2019).
Benkoe, T. et al. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PLoS One 8, e58720 (2013).
Weitkamp, J. H. et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut 62, 73–82 (2013).
Lee, Y. H. et al. Maternal bacterial infection during pregnancy and offspring’s risk of psychoses: variation by severity of infection and offspring sex. Am. J. Psychiatry 177, 66–75 (2020).
Kaushic, C., Richardson, J. M. & Wira, C. R. Regulation of polymeric immunoglobulin A receptor messenger ribonucleic acid expression in rodent uteri: effect of sex hormones. Endocrinology 136, 2836–2844 (1995).
Kaetzel, C. S. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 206, 83–99 (2005).
Acknowledgements
The authors wish to thank Sera Sebastian and Maria Melheim at the Oslo University Hospital Rikshospitalet, Oslo, Norway, for their contribution with the animal experiments and collection of the samples.
Funding
This study was part of the research program of the Postgraduate School for Behavioural and Cognitive Neurosciences (BCN), University of Groningen, Groningen, The Netherlands. B.M.D. was financially supported by a Junior Scientific Master Class grant of the University of Groningen. M.B. was financially supported by the China Scholarship Council.
Author information
Authors and Affiliations
Contributions
Each author has met the Pediatric Research authorship requirements. Substantial contributions to conception and design: B.M.D., M.B., R.S., O.D.S., J.B.F.H., A.F.B., T.P., E.M.W.K. Analysis and interpretation of data: B.M.D., M.B., T.P., E.M.W.K. Drafting the article or revising it critically for important intellectual content: B.M.D., M.B., J.B.F.H., A.F.B., T.P., E.M.W.K. Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dotinga, B.M., Bao, M., Solberg, R. et al. Gene expression in the intestine of newborn piglets after hypoxia-reoxygenation. Pediatr Res 94, 1365–1372 (2023). https://doi.org/10.1038/s41390-023-02657-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02657-4