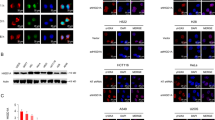
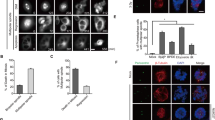
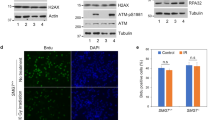
Abstract
The Rad9–Rad1–Hus1 (9-1-1) cell cycle checkpoint complex plays a key role in the DNA damage response. Cells with a defective 9-1-1 complex have been shown to be sensitive to apoptosis induced by certain types of genotoxic stress. However, the mechanism linking the loss of a functional 9-1-1 complex to the cell death machinery has yet to be determined. Here, we report that etoposide treatment dramatically upregulates the BH3-only proteins, Bim and Puma, in Hus1-deficient cells. Inhibition of either Bim or Puma expression in Hus1-knockout cells confers significant resistance to etoposide-induced apoptosis, whereas knockdown of both proteins results in further resistance, suggesting that Bim and Puma cooperate in sensitizing Hus1-deficient cells to etoposide treatment. Moreover, we found that Rad9 collaborates with Bim and Puma to sensitize Hus1-deficient cells to etoposide-induced apoptosis. In response to DNA damage, Rad9 localizes to chromatin in Hus1-wild-type cells, whereas in Hus1-deficient cells, it is predominantly located in the cytoplasm where it binds to Bcl-2. Taken together, these results suggest that loss of Hus1 sensitizes cells to etoposide-induced apoptosis not only by inducing Bim and Puma expressions but also by releasing Rad9 into the cytosol to augment mitochondrial apoptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abraham RT . (2001). Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196.
Adams JM, Cory S . (2007). The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26: 1324–1337.
Bakkenist CJ, Kastan MB . (2004). Initiating cellular stress responses. Cell 118: 9–17.
Bao S, Lu T, Wang X, Zheng H, Wang LE, Wei Q et al. (2004). Disruption of the Rad9/Rad1/Hus1 (9-1-1) complex leads to checkpoint signaling and replication defects. Oncogene 23: 5586–5593.
Bermudez VP, Lindsey-Boltz LA, Cesare AJ, Maniwa Y, Griffith JD, Hurwitz J et al. (2003). Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc Natl Acad Sci USA 100: 1633–1638.
Brandt PD, Helt CE, Keng PC, Bambara RA . (2006). The Rad9 protein enhances survival and promotes DNA repair following exposure to ionizing radiation. Biochem Biophys Res Commun 347: 232–237.
Burtelow MA, Kaufmann SH, Karnitz LM . (2000). Retention of the human Rad9 checkpoint complex in extraction-resistant nuclear complexes after DNA damage. J Biol Chem 275: 26343–26348.
Burtelow MA, Roos-Mattjus PM, Rauen M, Babendure JR, Karnitz LM . (2001). Reconstitution and molecular analysis of the hRad9–hHus1–hRad1 (9-1-1) DNA damage-responsive checkpoint complex. J Biol Chem 276: 25903–25909.
Chen MJ, Lin YT, Lieberman HB, Chen G, Lee EY . (2001). ATM-dependent phosphorylation of human Rad9 is required for ionizing radiation-induced checkpoint activation. J Biol Chem 276: 16580–16586.
Cory S, Huang DC, Adams JM . (2003). The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22: 8590–8607.
Dang T, Bao S, Wang XF . (2005). Human Rad9 is required for the activation of S-phase checkpoint and the maintenance of chromosomal stability. Genes Cells 10: 287–295.
Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ . (2000). Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol 10: 1201–1204.
Galonek HL, Hardwick JM . (2006). Upgrading the BCL-2 network. Nat Cell Biol 8: 1317–1319.
Hershko T, Ginsberg D . (2004). Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem 279: 8627–8634.
Hirai I, Wang HG . (2002). A role of the C-terminal region of human Rad9 (hRad9) in nuclear transport of the hRad9 checkpoint complex. J Biol Chem 277: 25722–25727.
Hopkins KM, Auerbach W, Wang XY, Hande MP, Hang H, Wolgemuth DJ et al. (2004). Deletion of mouse rad9 causes abnormal cellular responses to DNA damage, genomic instability, and embryonic lethality. Mol Cell Biol 24: 7235–7248.
Huang DC, Strasser A . (2000). BH3-only proteins-essential initiators of apoptotic cell death. Cell 103: 839–842.
Ishii H, Inageta T, Mimori K, Saito T, Sasaki H, Isobe M et al. (2005). Frag1, a homolog of alternative replication factor C subunits, links replication stress surveillance with apoptosis. Proc Natl Acad Sci USA 102: 9655–9660.
Kinzel B, Hall J, Natt F, Weiler J, Cohen D . (2002). Downregulation of Hus1 by antisense oligonucleotides enhances the sensitivity of human lung carcinoma cells to cisplatin. Cancer 94: 1808–1814.
Komatsu K, Hopkins KM, Lieberman HB, Wang H-G . (2000a). Schizosaccharomyces pombe rad9 contains a BH3-like region and interacts with the anti-apoptotic protein bcl-2. FEBS Lett 481: 122–126.
Komatsu K, Miyashita T, Hang H, Hopkins KM, Zheng W, Cuddeback S et al. (2000b). Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis. Nat Cell Biol 2: 1–6.
Lee MW, Hirai I, Wang H-G . (2003). Caspase-3-mediated cleavage of Rad9 during apoptosis. Oncogene 22: 6340–6346.
Lieberman HB . (2006). Rad9, an evolutionarily conserved gene with multiple functions for preserving genomic integrity. J Cell Biochem 97: 690–697.
Lindsey-Boltz LA, Bermudez VP, Hurwitz J, Sancar A . (2001). Purification and characterization of human DNA damage checkpoint Rad complexes. Proc Natl Acad Sci USA 98: 11236–11241.
Loegering D, Arlander SJ, Hackbarth J, Vroman BT, Roos-Mattjus P, Hopkins KM et al. (2004). Rad9 protects cells from topoisomerase poison-induced cell death. J Biol Chem 279: 18641–18647.
Melo J, Toczyski D . (2002). A unified view of the DNA-damage checkpoint. Curr Opin Cell Biol 14: 237–245.
Mendez J, Stillman B . (2000). Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol 20: 8602–8612.
Montecucco A, Biamonti G . (2007). Cellular response to etoposide treatment. Cancer Lett 252: 9–18.
Nakano K, Vousden KH . (2001). PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7: 683–694.
Niida H, Nakanishi M . (2006). DNA damage checkpoints in mammals. Mutagenesis 21: 3–9.
Oltvai ZN, Korsmeyer SJ . (1994). Checkpoints of dueling dimers foil death wishes. Cell 79: 189–192.
Parrilla-Castellar ER, Arlander SJ, Karnitz L . (2004). Dial 9-1-1 for DNA damage: the Rad9–Hus1–Rad1 (9-1-1) clamp complex. DNA Repair (Amst) 3: 1009–1014.
Puthalakath H, Strasser A . (2002). Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 9: 505–512.
Rauen M, Burtelow MA, Dufault VM, Karnitz LM . (2000). The human checkpoint protein hRad17 interacts with the PCNA-like proteins hRad1, hHus1, and hRad9. J Biol Chem 275: 29767–29771.
Shiloh Y . (2003). ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168.
Strasser A . (2005). The role of BH3-only proteins in the immune system. Nat Rev Immunol 5: 189–200.
Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA et al. (2003). FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem 278: 49795–49805.
Volkmer E, Karnitz LM . (1999). Human homologs of Schizosaccharomyces pombe rad1, hus1, and rad9 form a DNA damage-responsive protein complex. J Biol Chem 274: 567–570.
Wang H-G, Rapp UR, Reed JC . (1996). Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87: 629–638.
Wang X, Zou L, Zheng H, Wei Q, Elledge SJ, Li L . (2003). Genomic instability and endoreduplication triggered by RAD17 deletion. Genes Dev 17: 965–970.
Weiss RS, Enoch T, Leder P . (2000). Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev 14: 1886–1898.
Weiss RS, Leder P, Vaziri C . (2003). Critical role for mouse Hus1 in an S-phase DNA damage cell cycle checkpoint. Mol Cell Biol 23: 791–803.
Weiss RS, Matsuoka S, Elledge SJ, Leder P . (2002). Hus1 acts upstream of chk1 in a mammalian DNA damage response pathway. Curr Biol 12: 73–77.
Willis SN, Adams JM . (2005). Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 17: 617–625.
Wong HK, Fricker M, Wyttenbach A, Villunger A, Michalak EM, Strasser A et al. (2005). Mutually exclusive subsets of BH3-only proteins are activated by the p53 and c-Jun N-terminal kinase/c-Jun signaling pathways during cortical neuron apoptosis induced by arsenite. Mol Cell Biol 25: 8732–8747.
Yang JY, Xia W, Hu MC . (2006). Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int J Oncol 29: 643–648.
Yoshida K, Komatsu K, Wang HG, Kufe D . (2002). c-Abl tyrosine kinase regulates the human Rad9 checkpoint protein in response to DNA damage. Mol Cell Biol 22: 3292–3300.
Yoshida K, Wang HG, Miki Y, Kufe D . (2003). Protein kinase Cdelta is responsible for constitutive and DNA damage-induced phosphorylation of Rad9. EMBO J 22: 1431–1441.
Zhou BB, Elledge SJ . (2000). The DNA damage response: putting checkpoints in perspective. Nature 408: 433–439.
Zou L, Cortez D, Elledge SJ . (2002). Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev 16: 198–208.
Acknowledgements
We thank the Analytical Microscopy Core facility at the H Lee Moffitt Cancer Center and Research Institute for their technical assistance. This work was supported by grants from the National Institutes of Health (CA90315 to H-GW and CA108773 to RSW) and the Department of Defense Breast Cancer Research Program (BC050563 to CLM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Meyerkord, C., Takahashi, Y., Araya, R. et al. Loss of Hus1 sensitizes cells to etoposide-induced apoptosis by regulating BH3-only proteins. Oncogene 27, 7248–7259 (2008). https://doi.org/10.1038/onc.2008.336
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.336
Keywords
This article is cited by
-
ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands
The EMBO Journal (2009)