Abstract
Repulsive guidance molecules (RGMs) control crucial processes including cell motility, adhesion, immune-cell regulation and systemic iron metabolism. RGMs signal via the neogenin (NEO1) and the bone morphogenetic protein (BMP) pathways. Here, we report crystal structures of the N-terminal domains of all human RGM family members in complex with the BMP ligand BMP2, revealing a new protein fold and a conserved BMP-binding mode. Our structural and functional data suggest a pH-linked mechanism for RGM-activated BMP signaling and offer a rationale for RGM mutations causing juvenile hemochromatosis. We also determined the crystal structure of the ternary BMP2–RGM–NEO1 complex, which, along with solution scattering and live-cell super-resolution fluorescence microscopy, indicates BMP-induced clustering of the RGM–NEO1 complex. Our results show how RGM acts as the central hub that links BMP and NEO1 and physically connects these fundamental signaling pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
14 May 2015
In the version of this supplementary file originally posted online on 4 May 2015, the title and legend of Supplementary Figure 7 reproduced part of the title and legend from Supplementary Figure 6, and descriptions of panels c–f were missing. The errors have been corrected in this file as of 14 May 2015.
References
Yamashita, T., Mueller, B.K. & Hata, K. Neogenin and repulsive guidance molecule signaling in the central nervous system. Curr. Opin. Neurobiol. 17, 29–34 (2007).
Mirakaj, V. et al. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc. Natl. Acad. Sci. USA 108, 6555–6560 (2011).
Muramatsu, R. et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat. Med. 17, 488–494 (2011).
Li, V.S. et al. Frequent inactivation of axon guidance molecule RGMA in human colon cancer through genetic and epigenetic mechanisms. Gastroenterology 137, 176–187 (2009).
Papanikolaou, G. et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat. Genet. 36, 77–82 (2004).
Monnier, P.P. et al. RGM is a repulsive guidance molecule for retinal axons. Nature 419, 392–395 (2002).
Rajagopalan, S. et al. Neogenin mediates the action of repulsive guidance molecule. Nat. Cell Biol. 6, 756–762 (2004).
Matsunaga, E. & Chedotal, A. Repulsive guidance molecule/neogenin: a novel ligand-receptor system playing multiple roles in neural development. Dev. Growth Differ. 46, 481–486 (2004).
Bell, C.H. et al. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science 341, 77–80 (2013).
Babitt, J.L. et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet. 38, 531–539 (2006).
Samad, T.A. et al. DRAGON, a bone morphogenetic protein co-receptor. J. Biol. Chem. 280, 14122–14129 (2005).
Babitt, J.L. et al. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J. Biol. Chem. 280, 29820–29827 (2005).
Lee, D.H. et al. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood 115, 3136–3145 (2010).
Zhang, A.S., Yang, F., Wang, J., Tsukamoto, H. & Enns, C.A. Hemojuvelin-neogenin interaction is required for bone morphogenic protein-4-induced hepcidin expression. J. Biol. Chem. 284, 22580–22589 (2009).
Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 13, 616–630 (2012).
Sieber, C., Kopf, J., Hiepen, C. & Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 20, 343–355 (2009).
Bragdon, B. et al. Bone morphogenetic proteins: a critical review. Cell. Signal. 23, 609–620 (2011).
Shi, Y. & Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Feng, X.H. & Derynck, R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 21, 659–693 (2005).
Derynck, R. & Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Di Guglielmo, G.M., Le Roy, C., Goodfellow, A.F. & Wrana, J.L. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 5, 410–421 (2003).
Hartung, A. et al. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol. Cell. Biol. 26, 7791–7805 (2006).
Heining, E., Bhushan, R., Paarmann, P., Henis, Y.I. & Knaus, P. Spatial segregation of BMP/Smad signaling affects osteoblast differentiation in C2C12 cells. PLoS ONE 6, e25163 (2011).
Alborzinia, H. et al. Quantitative kinetics analysis of BMP2 uptake into cells and its modulation by BMP antagonists. J. Cell Sci. 126, 117–127 (2013).
Shi, W. et al. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J. Cell Sci. 120, 1216–1224 (2007).
Brazil, D.P., Church, R.H., Surae, S., Godson, C. & Martin, F. BMP signalling: agony and antagony in the family. Trends Cell Biol. 25, 249–264 (2015).
Xia, Y. et al. Dragon (repulsive guidance molecule b) inhibits IL-6 expression in macrophages. J. Immunol. 186, 1369–1376 (2011).
Lee, P.L., Beutler, E., Rao, S.V. & Barton, J.C. Genetic abnormalities and juvenile hemochromatosis: mutations of the HJV gene encoding hemojuvelin. Blood 103, 4669–4671 (2004).
Lok, C.Y. et al. Iron overload in the Asian community. Blood 114, 20–25 (2009).
Parrow, N.L. & Fleming, R.E. Bone morphogenetic proteins as regulators of iron metabolism. Annu. Rev. Nutr. 34, 77–94 (2014).
Yang, F., West, A.P. Jr., Allendorph, G.P., Choe, S. & Bjorkman, P.J. Neogenin interacts with hemojuvelin through its two membrane-proximal fibronectin type III domains. Biochemistry 47, 4237–4245 (2008).
Wu, Q., Sun, C.C., Lin, H.Y. & Babitt, J.L. Repulsive guidance molecule (RGM) family proteins exhibit differential binding kinetics for bone morphogenetic proteins (BMPs). PLoS ONE 7, e46307 (2012).
Xiong, J.P. et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 296, 151–155 (2002).
Camus, L.M. & Lambert, L.A. Molecular evolution of hemojuvelin and the repulsive guidance molecule family. J. Mol. Evol. 65, 68–81 (2007).
Kuns-Hashimoto, R., Kuninger, D., Nili, M. & Rotwein, P. Selective binding of RGMc/hemojuvelin, a key protein in systemic iron metabolism, to BMP-2 and neogenin. Am. J. Physiol. Cell Physiol. 294, C994–C1003 (2008).
Roetto, A. et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat. Genet. 33, 21–22 (2003).
Weinstein, D.A. et al. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood 100, 3776–3781 (2002).
Pagani, A., Silvestri, L., Nai, A. & Camaschella, C. Hemojuvelin N-terminal mutants reach the plasma membrane but do not activate the hepcidin response. Haematologica 93, 1466–1472 (2008).
Allendorph, G.P., Vale, W.W. & Choe, S. Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc. Natl. Acad. Sci. USA 103, 7643–7648 (2006).
Weber, D. et al. A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct. Biol. 7, 6 (2007).
Townson, S.A. et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J. Biol. Chem. 287, 27313–27325 (2012).
Heinecke, K. et al. Receptor oligomerization and beyond: a case study in bone morphogenetic proteins. BMC Biol. 7, 59 (2009).
Keller, S., Nickel, J., Zhang, J.-L., Sebald, W. & Mueller, T.D. Molecular recognition of BMP-2 and BMP receptor IA. Nat. Struct. Mol. Biol. 11, 481–488 (2004).
Korchynskyi, O. & ten Dijke, P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 277, 4883–4891 (2002).
Rossy, J., Owen, D.M., Williamson, D.J., Yang, Z. & Gaus, K. Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nat. Immunol. 14, 82–89 (2013).
Zhou, Z. et al. Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev. Cell 19, 90–102 (2010).
Xia, Y., Babitt, J.L., Sidis, Y., Chung, R.T. & Lin, H.Y. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood 111, 5195–5204 (2008).
Tassew, N.G. et al. Modifying lipid rafts promotes regeneration and functional recovery. Cell Reports 8, 1146–1159 (2014).
Vieira, A.V., Lamaze, C. & Schmid, S.L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274, 2086–2089 (1996).
Le Roy, C. & Wrana, J.L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112–126 (2005).
Bell, C.H. et al. Structure of the repulsive guidance molecule (RGM)–neogenin signaling hub. Science 341, 77–80 (2013).
Aricescu, A.R., Lu, W. & Jones, E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 (2006).
Zhao, Y. et al. Automation of large scale transient protein expression in mammalian cells. J. Struct. Biol. 175, 209–215 (2011).
Chang, V.T. et al. Glycoprotein structural genomics: solving the glycosylation problem. Structure 15, 267–273 (2007).
von Einem, S., Schwarz, E. & Rudolph, R. A novel TWO-STEP renaturation procedure for efficient production of recombinant BMP-2. Protein Expr. Purif. 73, 65–69 (2010).
Trombetta, E.S. & Parodi, A.J. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 19, 649–676 (2003).
Walter, T.S. et al. A procedure for setting up high-throughput nanolitre crystallization experiments: crystallization workflow for initial screening, automated storage, imaging and optimization. Acta Crystallogr. D Biol. Crystallogr. 61, 651–657 (2005).
Mayo, C.J. et al. Benefits of automated crystallization plate tracking, imaging, and analysis. Structure 13, 175–182 (2005).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 (2006).
Kabsch, W. Automatic indexing of rotation diffraction patterns. J. Appl. Crystallogr. 21, 67–72 (1988).
Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 (1993).
Leslie, A.G.W. The integration of macromolecular diffraction data. Acta Crystallogr. D Biol. Crystallogr. 62, 48–57 (2006).
Sauter, N.K., Grosse-Kunstleve, R.W. & Adams, P.D. Robust indexing for automatic data collection. J. Appl. Crystallogr. 37, 399–409 (2004).
Zhang, Z., Sauter, N.K., van den Bedem, H., Snell, G. & Deacon, A.M. Automated diffraction image analysis and spot searching for high-throughput crystal screening. J. Appl. Crystallogr. 39, 112–119 (2006).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Scheufler, C., Sebald, W. & Hulsmeyer, M. Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J. Mol. Biol. 287, 103–115 (1999).
Cowtan, K. Recent developments in classical density modification. Acta Crystallogr. D Biol. Crystallogr. 66, 470–478 (2010).
Cowtan, K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 62, 1002–1011 (2006).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Davis, I.W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007).
Baker, N.A., Sept, D., Joseph, S., Holst, M.J. & McCammon, J.A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 98, 10037–10041 (2001).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Pernot, P. et al. Upgraded ESRF BM29 beamline for SAXS on macromolecules in solution. J. Synchrotron Radiat. 20, 660–664 (2013).
Petoukhov, M.V. et al. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Crystallogr. 45, 342–350 (2012).
Rambo, R.P. & Tainer, J.A. Accurate assessment of mass, models and resolution by small-angle scattering. Nature 496, 477–481 (2013).
Leaver-Fay, A. et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 487, 545–574 (2011).
Eswar, N. et al. Tools for comparative protein structure modeling and analysis. Nucleic Acids Res. 31, 3375–3380 (2003).
Weinkam, P., Pons, J. & Sali, A. Structure-based model of allostery predicts coupling between distant sites. Proc. Natl. Acad. Sci. USA 109, 4875–4880 (2012).
Pelikan, M., Hura, G.L. & Hammel, M. Structure and flexibility within proteins as identified through small angle X-ray scattering. Gen. Physiol. Biophys. 28, 174–189 (2009).
Schneidman-Duhovny, D., Hammel, M., Tainer, J.A. & Sali, A. Accurate SAXS profile computation and its assessment by contrast variation experiments. Biophys. J. 105, 962–974 (2013).
Guttman, M., Weinkam, P., Sali, A. & Lee, K.K. All-atom ensemble modeling to analyze small-angle x-ray scattering of glycosylated proteins. Structure 21, 321–331 (2013).
Johnsson, B., Löfås, S. & Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 198, 268–277 (1991).
Herrera, B. & Inman, G.J. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins: identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 10, 20 (2009).
Korchynskyi, O. & ten Dijke, P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 277, 4883–4891 (2002).
Perry, G.L.W. SpPack: spatial point pattern analysis in Excel using Visual Basic for Applications (VBA). Environ. Model. Softw. 19, 559–569 (2004).
Rossy, J., Owen, D.M., Williamson, D.J., Yang, Z. & Gaus, K. Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nat. Immunol. 14, 82–89 (2013).
Owen, D.M. et al. PALM imaging and cluster analysis of protein heterogeneity at the cell surface. J Biophotonics 3, 446–454 (2010).
Owen, D.M., Williamson, D., Magenau, A. & Gaus, K. Optical techniques for imaging membrane domains in live cells (live-cell palm of protein clustering). Methods Enzymol. 504, 221–235 (2012).
Williamson, D.J. et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat. Immunol. 12, 655–662 (2011).
Acknowledgements
We thank the staff of beamlines I03, I04 and I04-1 at the Diamond Light Source (X-ray diffraction data, proposal MX-10627), BM29 at the European Synchrotron Radiation Facility (SAXS data) and the Cellular Imaging Core at the Wellcome Trust Centre for Human Genetics (TIRF and dSTORM data) for assistance; T. Walter and K. Harlos for help with crystallization; R. Robinson and G. Sutton for help with MALS; and A.R. Aricescu and D.I. Stuart for reading the manuscript. This work was supported by Cancer Research UK (C20724/A14414 (C.S.)) and the Wellcome Trust (097301/Z/11/Z (E.G.H.)). Further support from the Wellcome Trust core award grant 090532/Z/09/Z (C.S.) and the Wellcome Trust multi-user equipment grant 101584MA (S.P.-P. and C.S.) are acknowledged. E.G.H. is funded by a Wellcome Trust PhD Studentship. J.E. is supported as a Marie-Curie Postdoctoral Fellow (FP7-328531). S.P.-P. is supported as a Nuffield Department of Medicine Leadership Fellow. C.S. is supported as a Cancer Research UK Senior Research Fellow.
Author information
Authors and Affiliations
Contributions
C.S. designed and supervised the project. E.G.H. and C.H.B. cloned all RGM, NEO1 and BMP constructs. E.G.H., B.B. and C.H.B. performed protein expression and purification, and E.G.H. crystallized the proteins. E.G.H. and C.S. collected the data and solved and refined the crystal structures. E.G.H. and B.B. carried out SPR and luciferase experiments, and E.G.H. performed the MALS experiments. SAXS data were collected by J.E. and E.G.H., and J.E. conducted all subsequent SAXS data processing. E.G.H., B.B. and S.P.-P. collected the imaging data, and S.P.-P. completed the dSTORM data processing. C.S. and E.G.H. wrote the paper, and all authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Sequence alignments of BMP and RGM family members.
(a) Sequence alignment of human BMP family members. Numbering corresponds to the full length human BMP2; residues of BMP2 molecule 1 forming hydrophilic interactions to RGMC are highlighted in dark blue and non-bonded contacts in light blue. Residues of BMP2 molecule 2 forming non-bonded contacts are shown in red. Disulfide bridges are indicated by Roman Numerals. (b) Sequence alignment of the N-terminal domain of RGM family members. Numbering is that of the full length human RGMC. Secondary structure assignments for RGMAND (blue), RGMBND (yellow) and RGMCND (orange) are displayed above the alignment. RGM residues forming hydrophilic interactions with BMP2 are highlighted in dark blue, exclusively formed by RGMA in salmon and exclusively formed by RGMC in green. Non-bonded contact residues interacting with BMP2 are depicted in magenta. Disulfide bridges are indicated by Roman Numerals. Asterisks (*) indicate disease-related residues identified in human RGMC. hRGM: human RGM, mRGM: mouse RGM, zRGM: zebrafish RGM, xRGM: Xenopus laevis RGM.
Supplementary Figure 2 Electron density maps of the RGMC–BMP2 complex.
(a) Initial electron density map, at 2.35 Å resolution, of the RGMCND–BMP2 complex after molecular replacement (using only the BMP2 dimer as search model) in PHASER (McCoy, A. J. et al. (2007) J Appl Crystallogr 40, 658-674) and density modification in PARROT (Cowtan, K. (2010) Acta Crystallogr D 66, 470-478). Contour level is 1.0 σ. The final refined RGMCND–BMP2 model is represented as ribbon (RGMC: blue and green; BMP2: yellow and magenta). (b-c) Close-up view onto the RGMC–BMP2 interface. Protein chains are depicted in stick representation. Color coding is as in (a). (b) Initial electron density map as described in (a). (c) SigmaA-weighted 2FO-FC map after the final round of refinement from autoBUSTER (BUSTER, version 2.8.0., Cambridge, 2011) contoured at 1.0 σ.
Supplementary Figure 3 RGMCND structure and comparison.
(a) Topology diagram of RGMCND (adapted from PDBSUM (http://www.ebi.ac.uk/pdbsum/)) in rainbow coloring (N-terminus: blue; C-terminus: red). N- and C-termini and residue numbers are shown. (b) Cartoon representation of the RGMCND structure. Color coding is as in (a). RGMND forms a compact three helix bundle fold, stabilized by three disulfide bonds, supported by a hydrophobic core including residues from all three helices, depicted as sticks. Right panel rotated 90° around the x-axis compared to left panel. Disulfide bridges are numbered in Roman numerals. The disordered α1–α2 loop is shown as a dotted line. (c-g) Structural comparison of RGMCND using the PDBefold server (http://www.ebi.ac.uk/msd-srv/ssm/). RGMCND ((c), cyan) shares the closest structural similarity to the human MTCP1 oncogene ((d), pdb 2HP8 (Barthe, P. et al. (1997) J Mol Biol 274, 801-815), slate; r.m.s.d. of 2.78 Å for 60 equivalent Cα positions, sequence identity: 5%), the MIT domain of VPS4-like ATPases ((e), pdb 2V6Y (Obita, T. et al. (2007) Nature 449, 735-739), magenta, r.m.s.d. of 2.47 Å for 60 equivalent Cα positions, sequence identity: 3%) and the complement inhibitor EHP from S. aureus ((f), pdb 2NOJ (Hammel, M. et al. (2007). J Biol Chem 282, 30051-30061), yellow, r.m.s.d. of 2.34 Å for 54 equivalent Cα positions, sequence identity: 6%). The position of disulfide bridge II in RGMND is conserved in MTCP1 and highlighted by an asterisk. A structural superposition is shown in (g). The two views are related by a rotation of 90° around the x-axis.
Supplementary Figure 4 SPR equilibrium binding experiments.
(a-l) Binding of different constructs and mutants of RGMA, RGMB and RGMC, respectively, to BMP2. (m-r) Binding of BMP receptor ectodomain constructs to BMP2 ligand (m-o) or to eRGMB (p-r). For (k) the eRGMB-NEO1FN56 complex was purified via size exclusion chromatography prior to SPR. Graphs show a plot of the equilibrium binding response (response units (RU)) against concentration of the used analytes. All experiments were performed in duplicate. Binding constants (Kd) are given as mean with the error representing the standard error of the mean (n=2 technical repeats) Fits are shown as lines. Sensorgrams are shown..Binding constants (Kd), Bmax and surface response units (Bsurface) are indicated below the graphs.
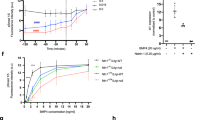
Supplementary Figure 5 Analysis of the effects of eBMPR1A and eRGMB on SMAD-mediated transcriptional activation by BMP-responsive reporter (BRE-LUC).
(a-c) LLC-PK1 cells (a, b) or C2C12 cells (c) were stimulated with either buffer control or different concentrations of BMP2: 25 nM (a), 6 nM BMP2 (b); or 10 nM BMP2 (c). BMP2 was pre-incubated with 0.4, 1.6, 6.3, 25 or 100 X molar excess of eBMPR1A or eRGMB. Average BRE-Luc relative response was calculated for each condition from two independent experiments. For (a): Control n = 4, +25 nM BMP2 n = 12, all others n= 8. For (b): Control and + 6 nM BMP2 n = 33, all eBMPR1A n = 41, all eRGMB n = 32. For (c): Control n= 6, + 10 nM BMP2 n = 18, all others n = 12. Where n = cell cultures. Error bars are s.e.m., dotted line indicates average increase in relative luciferase response induced by the respective BMP2 concentration in each case.
Supplementary Figure 6 Interface analysis and comparison of the ternary RGMB–NEO1–BMP2 complex to the binary RGMB–NEO1 and RGMB–BMP2 complexes.
(a) Cartoon representations of the ternary RGMB–NEO1–BMP2 complex (grey) and superimposed binary RGMB–NEO1 (orange and red) and RGMB–BMP2 (orange and blue) complexes. Superposition of the binary RGMBND–BMP2 complex from this study yields in an r.m.s.d. of 0.799 Å for 328 equivalent Cα positions. Using the previously determined structure of the binary NEO1FN56–eRGMB complex (pdb 4BQ6, site-1 interface (Bell, C. H. et al. (2013) Science 341(6141), 77-80) an r.m.s.d. of 0.511 Å for 368 equivalent Cα positions was achieved when compared with the ternary RGMB–NEO1–BMP2 complex. (b) Table showing the analysis of the interfaces highlighted in (a). Whereas the binary BMP2–BMP2, BMP2–RGMBND and NEO1FN56–RGMBCD interfaces show values expected for a physiological interaction, the BMP2-NEO1FN5 interface has a at least 3-times smaller buried interface area and significantly lower score. ashape complementary calculated with program sc (Lawrence M. C. et al. (1993) J Mol Biol 234, 946-950); btotal buried surface area (from PISA (Krissinel E. et al. (2007) J Mol Biol 372, 774-797 (1)); csolvation free energy gain upon formation of the interface1; dnumber of interfacing residues in the complex1; enumber of potential hydrogen bonds across the interface1; fcomplexation Significance Score indicating how significant the interface is for assembly formation1; gbrackets indicate the percentage of buried interface area compared to the overall surface of the free molecules; hthe BMP2-BMP2 interface also includes a intermolecular disulfide bridge that is not included in the number of interacting residues.
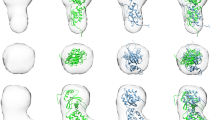
Supplementary Figure 7 SAXS solution structures of NEO1FN56M, eRGMB, eRGMB–NEO1FN56M and BMP2–eRGMB–NEO1FN56M.
(a-b) SEC of the BMP2-eRGMB-NEO1FN56M complex formed by saturating BMP2 and eRGMB with a molar excess of NEO1FN56M. The SEC elution profile (a) and the corresponding SDS-PAGE of the fractions under reducing conditions (b) are shown. The first peak (I) corresponds to the ternary BMP2-eRGMB-NEO1FN56M complex, the second (II) to the binary eRGMB-NEO1FN56M complex, and the third (III) to uncomplexed NEO1FN56M, which was added in a 1.5 molar excess. MW: Sigma Molecular Weight Marker S8445. The asterisks mark the two RGM products resulting from autocatalytic cleavage7. (c-f) SAXS analysis of NEO1FN56M (c), eRGMB (d), eRGMB-NEO1FN56M (e) and BMP2-eRGMB-NEO1FN56M (f) after isolation by SEC. Experimental scattering curves (black) and calculated scattering patterns (red) are shown to a maximal momentum transfer of q = 0.35 Å−1. The fitting residuals of the experimental scattering curves and calculated scattering patterns are displayed in the lower left insets. The upper right insets show the experimental (black) and calculated (red) normalized pair distance distribution (P(r)) function. The lower right insets display the experimental (black) and calculated (red) Guinier region. The shaded area indicates the range of fitting for RG analysis (RG•S ≤ 1.3). The fit of the calculated scattering pattern (χ2), the derived maximum intra-particle diameter (DMAX), the radius of gyration (RG), and the molecular weight derived from the volume of correlation metric VC (MWVc) are annotated.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 32807 kb)
Rights and permissions
About this article
Cite this article
Healey, E., Bishop, B., Elegheert, J. et al. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat Struct Mol Biol 22, 458–465 (2015). https://doi.org/10.1038/nsmb.3016
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3016
This article is cited by
-
Transcriptomic characterization of the molecular mechanisms induced by RGMa during skeletal muscle nuclei accretion and hypertrophy
BMC Genomics (2022)
-
Atheroprone fluid shear stress-regulated ALK1-Endoglin-SMAD signaling originates from early endosomes
BMC Biology (2022)
-
RNF4~RGMb~BMP6 axis required for osteogenic differentiation and cancer cell survival
Cell Death & Disease (2022)
-
Netrin-1 functions as a suppressor of bone morphogenetic protein (BMP) signaling
Scientific Reports (2021)
-
RGMa can induce skeletal muscle cell hyperplasia via association with neogenin signalling pathway
In Vitro Cellular & Developmental Biology - Animal (2021)