Abstract
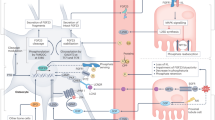
Fibroblast growth factor 23 (FGF23) was initially characterized as an important regulator of phosphate and calcium homeostasis. New research advances demonstrate that FGF23 is also linked to iron economy, inflammation and erythropoiesis. These advances have been fuelled, in part, by the serendipitous development of two distinct FGF23 assays that can substitute for invasive bone biopsies to infer the activity of the three main steps of FGF23 regulation in bone: transcription, post-translational modification and peptide cleavage. This ‘liquid bone biopsy for FGF23 dynamics’ enables large-scale longitudinal studies of FGF23 regulation that would otherwise be impossible in humans. The balance between FGF23 production, post-translational modification and cleavage is maintained or perturbed in different hereditary monogenic conditions and in acquired conditions that mimic these genetic disorders, including iron deficiency, inflammation, treatment with ferric carboxymaltose and chronic kidney disease. Looking ahead, a deeper understanding of the relationships between FGF23 regulation, iron homeostasis and erythropoiesis can be leveraged to devise novel therapeutic targets for treatment of anaemia and states of FGF23 excess, including chronic kidney disease.
Key points
-
The development of two complementary assays of full-length and C-terminal FGF23 provided tools to non-invasively survey FGF23 transcription and cleavage.
-
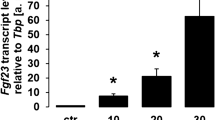
Iron deficiency, inflammation and erythropoiesis stimulate simultaneous production and cleavage of FGF23. This coupling of FGF23 transcription and FGF23 cleavage results in minimal change in concentrations of biologically active FGF23.
-
Albeit through different mechanisms, autosomal dominant hypophosphataemic rickets, chronic kidney disease and administration of ferric carboxymaltose reduce FGF23 cleavage. Uncoupling FGF23 protein cleavage from FGF23 gene transcription results in elevated concentrations of biologically active FGF23.
-
In individuals with normal kidney function, states of uncoupled FGF23 transcription and cleavage can lead to severe hypophosphataemia owing to the phosphaturic and vitamin D-suppressing effects of the increased levels of biologically active FGF23.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jonsson, K. B. et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348, 1656–1663 (2003).
Weber, T. J., Liu, S., Indridason, O. S. & Quarles, L. D. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J. Bone Miner. Res. 18, 1227–1234 (2003).
Scialla, J. J. et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 25, 349–360 (2014).
Nowak, K. L. et al. Fibroblast growth factor 23 and the risk of infection-related hospitalization in older adults. J. Am. Soc. Nephrol. 28, 1239–1246 (2017).
Garland, J. S. et al. Insulin resistance is associated with fibroblast growth factor-23 in stage 3-5 chronic kidney disease patients. J. Diabetes Complications 28, 61–65 (2014).
Shigematsu, T. et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am. J. Kidney Dis. 44, 250–256 (2004).
Hasegawa, H. et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78, 975–980 (2010).
Gutierrez, O. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2205–2215 (2005).
Farrow, E. G. et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc. Natl Acad. Sci. USA 108, E1146–E1155 (2011).
Wolf, M., Koch, T. A. & Bregman, D. B. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J. Bone Miner. Res. 28, 1793–1803 (2013).
Braithwaite, V., Jones, K. S., Assar, S., Schoenmakers, I. & Prentice, A. Predictors of intact and C-terminal fibroblast growth factor 23 in Gambian children. Endocr. Connect. 3, 1–10 (2014).
Holecki, M. et al. Inflammation but not obesity or insulin resistance is associated with increased plasma fibroblast growth factor 23 concentration in the elderly. Clin. Endocrinol. 82, 900–909 (2015).
Dounousi, E. et al. Intact FGF23 and alpha-klotho during acute inflammation/sepsis in CKD patients. Eur. J. Clin. Invest. 47, 470–472 (2017).
Munoz Mendoza, J. et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin. J. Am. Soc. Nephrol. 7, 1155–1162 (2012).
Clinkenbeard, E. L. et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J. Bone Miner. Res. 29, 361–369 (2014).
Coe, L. M. et al. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J. Biol. Chem. 289, 9795–9810 (2014).
Clinkenbeard, E. L. et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 102, e427–e430 (2017).
Toro, L. et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int. 93, 1131–1141 (2018).
Hanudel, M. R. et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfy189 (2018).
David, V. et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 89, 135–146 (2016).
ADHR Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 26, 345–348 (2000).
Wolf, M. et al. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight 3, 124486 (2018).
Bozentowicz-Wikarek, M. et al. C-terminal to intact fibroblast growth factor 23 ratio in relation to estimated glomerular filtration rate in elderly population. Kidney Blood Press. Res. 41, 519–526 (2016).
Yoshiko, Y. et al. Mineralized tissue cells are a principal source of FGF23. Bone 40, 1565–1573 (2007).
Leifheit-Nestler, M. et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol. Dial. Transplant. 31, 1088–1099 (2016).
Sugiura, H. et al. Fibroblast growth factor 23 is upregulated in the kidney in a chronic kidney disease rat model. PLOS ONE 13, e0191706 (2018).
Smith, E. R., Tan, S. J., Holt, S. G. & Hewitson, T. D. FGF23 is synthesised locally by renal tubules and activates injury-primed fibroblasts. Sci. Rep. 7, 3345 (2017).
Bansal, S. et al. Spleen contributes significantly to increased circulating levels of fibroblast growth factor 23 in response to lipopolysaccharide-induced inflammation. Nephrol. Dial. Transplant. 32, 960–968 (2017).
van Venrooij, N. A. et al. FGF23 protein expression in coronary arteries is associated with impaired kidney function. Nephrol. Dial. Transplant. 29, 1525–1532 (2014).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001).
Wolf, M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 82, 737–747 (2012).
Erben, R. G. Update on FGF23 and Klotho signaling. Mol. Cell. Endocrinol. 432, 56–65 (2016).
Gattineni, J. et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am. J. Physiol. Ren. Physiol. 297, F282–F291 (2009).
Chen, G. et al. αKlotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553, 461–466 (2018).
Bai, X. et al. CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. J. Clin. Invest. 126, 667–680 (2016).
Koizumi, M., Komaba, H. & Fukagawa, M. Parathyroid function in chronic kidney disease: role of FGF23-Klotho axis. Contrib. Nephrol. 180, 110–123 (2013).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Krajisnik, T. et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 195, 125–131 (2007).
Rodriguez-Ortiz, M. E. et al. Calcium deficiency reduces circulating levels of FGF23. J. Am. Soc. Nephrol. 23, 1190–1197 (2012).
David, V. et al. Calcium regulates FGF-23 expression in bone. Endocrinology 154, 4469–4482 (2013).
Yuan, Q. et al. PTH ablation ameliorates the anomalies of Fgf23-deficient mice by suppressing the elevated vitamin D and calcium levels. Endocrinology 152, 4053–4061 (2011).
Kawata, T. et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J. Am. Soc. Nephrol. 18, 2683–2688 (2007).
Meir, T. et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 86, 1106–1115 (2014).
Liu, S. et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J. Am. Soc. Nephrol. 17, 1305–1315 (2006).
Saito, H. et al. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J. Biol. Chem. 280, 2543–2549 (2005).
Shimada, T. et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Ren. Physiol. 289, F1088–F1095 (2005).
Yu, X., Sabbagh, Y., Davis, S. I., Demay, M. B. & White, K. E. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36, 971–977 (2005).
Kolek, O. I. et al. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1036–G1042 (2005).
Portale, A. A. et al. Disordered FGF23 and mineral metabolism in children with CKD. Clin. J. Am. Soc. Nephrol. 9, 344–353 (2014).
Hill, K. M. et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 83, 959–966 (2013).
Isakova, T. et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 79, 1370–1378 (2011).
Lindberg, K. et al. The kidney is the principal organ mediating klotho effects. J. Am. Soc. Nephrol. 25, 2169–2175 (2014).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Nakatani, T., Ohnishi, M. & Razzaque, M. S. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 23, 3702–3711 (2009).
Wolf, M. & White, K. E. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr. Opin. Nephrol. Hypertens. 23, 411–419 (2014).
Ito, N. et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol. Cell. Endocrinol. 399, 208–218 (2015).
Haussler, M. R. et al. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 92, 77–98 (2013).
Kaneko, I. et al. FGF23 gene regulation by 1,25-dihydroxyvitamin D: opposing effects in adipocytes and osteocytes. J. Endocrinol. 226, 155–166 (2015).
Barthel, T. K. et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J. Steroid Biochem. Mol. Biol. 103, 381–388 (2007).
Lanske, B. & Razzaque, M. S. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 86, 1072–1074 (2014).
Lavi-Moshayoff, V., Wasserman, G., Meir, T., Silver, J. & Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am. J. Physiol. Ren. Physiol. 299, F882–F889 (2010).
Xiao, L., Esliger, A. & Hurley, M. M. Nuclear fibroblast growth factor 2 (FGF2) isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. J. Bone Miner. Res. 28, 35–45 (2013).
Smith, R. C. et al. Circulating αKlotho influences phosphate handling by controlling FGF23 production. J. Clin. Invest. 122, 4710–4715 (2012).
Riminucci, M. et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Invest. 112, 683–692 (2003).
Yamashita, T., Yoshioka, M. & Itoh, N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 277, 494–498 (2000).
Stubbs, J. R. et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J. Am. Soc. Nephrol. 18, 2116–2124 (2007).
Sitara, D. et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 (2004).
Liu, S. et al. Pathogenic role of Fgf23 in Hyp mice. Am. J. Physiol. Endocrinol. Metab. 291, E38–E49 (2006).
Liu, S. et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 278, 37419–37426 (2003).
The HYP consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat. Genet. 11, 130–136 (1995).
Kato, K. et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 281, 18370–18377 (2006).
Shimada, T. et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143, 3179–3182 (2002).
Ichikawa, S. et al. Genetic rescue of glycosylation-deficient Fgf23 in the Galnt3 knockout mouse. Endocrinology 155, 3891–3898 (2014).
Tagliabracci, V. S. et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl Acad. Sci. USA 111, 5520–5525 (2014).
Yamazaki, Y. et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 87, 4957–4960 (2002).
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Wang, X. et al. Inactivation of a novel FGF23 regulator, FAM20C, leads to hypophosphatemic rickets in mice. PLOS Genet. 8, e1002708 (2012).
Rafaelsen, S. H. et al. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J. Bone Miner. Res. 28, 1378–1385 (2013).
Smith, E. R., Cai, M. M., McMahon, L. P. & Holt, S. G. Biological variability of plasma intact and C-terminal FGF23 measurements. J. Clin. Endocrinol. Metab. 97, 3357–3365 (2012).
Bergwitz, C. et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J. Clin. Endocrinol. Metab. 94, 4267–4274 (2009).
Yancovitch, A. et al. Novel mutations in GALNT3 causing hyperphosphatemic familial tumoral calcinosis. J. Bone Miner. Metab. 29, 621–625 (2011).
Shimada, T. et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J. Clin. Endocrinol. Metab. 95, 578–585 (2010).
Malluche, H. H., Langub, M. C. & Monier-Faugere, M. C. The role of bone biopsy in clinical practice and research. Kidney Int. Suppl. 73, S20–S25 (1999).
Burnett, S. M. et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J. Bone Miner. Res. 21, 1187–1196 (2006).
Durham, B. H., Joseph, F., Bailey, L. M. & Fraser, W. D. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann. Clin. Biochem. 44, 463–466 (2007).
Imel, E. A. et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J. Clin. Endocrinol. Metab. 91, 2055–2061 (2006).
Lopez, A., Cacoub, P., Macdougall, I. C. & Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 387, 907–916 (2016).
Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 373, 485–486 (2015).
Kassebaum, N. J. et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 123, 615–624 (2014).
McLean, E., Cogswell, M., Egli, I., Wojdyla, D. & de Benoist, B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr. 12, 444–454 (2009).
Imel, E. A. et al. Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone 86, 98–105 (2016).
Ali, F. N., Josefson, J., Mendez, A. J., Mestan, K. & Wolf, M. Cord blood ferritin and fibroblast growth factor-23 levels in neonates. J. Clin. Endocrinol. Metab. 101, 1673–1679 (2016).
Rousseau, A. F., Souberbielle, J. C., Delanaye, P., Damas, P. & Cavalier, E. Fibroblast growth factor 23 in acute burn patients: novel insights from an intact-form assay. Burns 42, 1082–1087 (2016).
di Giuseppe, R. et al. Potential predictors of plasma fibroblast growth factor 23 concentrations: cross-sectional analysis in the EPIC-Germany study. PLOS ONE 10, e0133580 (2015).
Imel, E. A. et al. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J. Clin. Endocrinol. Metab. 96, 3541–3549 (2011).
Eisenga, M. F. et al. C-terminal fibroblast growth factor 23, iron deficiency, and mortality in renal transplant recipients. J. Am. Soc. Nephrol. 28, 3639–3646 (2017).
Eser, B. et al. Fibroblast growth factor is associated to left ventricular mass index, anemia and low values of transferrin saturation. Nefrologia 35, 465–472 (2015).
Honda, H. et al. High fibroblast growth factor 23 levels are associated with decreased ferritin levels and increased intravenous iron doses in hemodialysis patients. PLOS ONE 12, e0176984 (2017).
Lewerin, C. et al. Low serum iron is associated with high serum intact FGF23 in elderly men: the Swedish MrOS study. Bone 98, 1–8 (2017).
Hanudel, M. R. et al. Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am. J. Physiol. Ren. Physiol. 311, F1369–F1377 (2016).
Girelli, D., Nemeth, E. & Swinkels, D. W. Hepcidin in the diagnosis of iron disorders. Blood 127, 2809–2813 (2016).
Weiss, G., Ganz, T. & Goodnough, L. T. Anemia of inflammation. Blood 133, 40–50 (2019).
Sato, H. et al. Serum fibroblast growth factor 23 (FGF23) in patients with rheumatoid arthritis. Intern. Med. 55, 121–126 (2016).
Hanks, L. J., Casazza, K., Judd, S. E., Jenny, N. S. & Gutierrez, O. M. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLOS ONE 10, e0122885 (2015).
El-Hodhod, M. A., Hamdy, A. M., Abbas, A. A., Moftah, S. G. & Ramadan, A. A. Fibroblast growth factor 23 contributes to diminished bone mineral density in childhood inflammatory bowel disease. BMC Gastroenterol. 12, 44 (2012).
Manghat, P. et al. Fibroblast growth factor-23 is associated with C-reactive protein, serum phosphate and bone mineral density in chronic kidney disease. Osteoporos. Int. 21, 1853–1861 (2010).
Han, X., Xiao, Z. & Quarles, L. D. Membrane and integrative nuclear fibroblastic growth factor receptor (FGFR) regulation of FGF-23. J. Biol. Chem. 290, 10447–10459 (2015).
Yamazaki, M. et al. Interleukin-1-induced acute bone resorption facilitates the secretion of fibroblast growth factor 23 into the circulation. J. Bone Miner. Metab. 33, 342–354 (2015).
Jelkmann, W. Molecular biology of erythropoietin. Intern. Med. 43, 649–659 (2004).
Rabadi, S., Udo, I., Leaf, D. E., Waikar, S. S. & Christov, M. Acute blood loss stimulates fibroblast growth factor 23 production. Am. J. Physiol. Ren. Physiol. 314, F132–F139 (2018).
Agoro, R. et al. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 32, 3752–3764 (2018).
Daryadel, A. et al. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflugers Arch. 470, 1569–1582 (2018).
Flamme, I., Ellinghaus, P., Urrego, D. & Kruger, T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLOS ONE 12, e0186979 (2017).
Haase, V. H. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 27, 41–53 (2013).
Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992 (1998).
Zhang, Q. et al. The hypoxia-inducible factor-1alpha activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 4, 16011 (2016).
Fukumoto, S. Targeting fibroblast growth factor 23 signaling with antibodies and inhibitors, is there a rationale? Front. Endocrinol. 9, 48 (2018).
Goetz, R. et al. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc. Natl Acad. Sci. USA 107, 407–412 (2010).
Econs, M. J. & McEnery, P. T. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J. Clin. Endocrinol. Metab. 82, 674–681 (1997).
Kapelari, K., Kohle, J., Kotzot, D. & Hogler, W. Iron supplementation associated with loss of phenotype in autosomal dominant hypophosphatemic rickets. J. Clin. Endocrinol. Metab. 100, 3388–3392 (2015).
Carpenter, T. O. et al. Burosumab therapy in children with X-linked hypophosphatemia. N. Engl. J. Med. 378, 1987–1998 (2018).
Roberts, M. A. et al. Effects of intravenous iron on fibroblast growth factor 23 (FGF23) in haemodialysis patients: a randomized controlled trial. BMC Nephrol. 17, 177 (2016).
Deger, S. M. et al. The effects of iron on FGF23-mediated Ca-P metabolism in CKD patients. Clin. Exp. Nephrol. 17, 416–423 (2013).
Braithwaite, V., Prentice, A. M., Doherty, C. & Prentice, A. FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation study. Int. J. Pediatr. Endocrinol. 2012, 27 (2012).
Fukao, W. et al. Oral versus intravenous iron supplementation for the treatment of iron deficiency anemia in patients on maintenance hemodialysis-effect on fibroblast growth factor-23 metabolism. J. Ren. Nutr. 28, 270–277, (2018).
Yamamoto, S., Okada, Y., Mori, H., Fukumoto, S. & Tanaka, Y. Fibroblast growth factor 23-related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern. Med. 51, 2375–2378 (2012).
Schouten, B. J., Hunt, P. J., Livesey, J. H., Frampton, C. M. & Soule, S. G. FGF23 elevation and hypophosphatemia after intravenous iron polymaltose: a prospective study. J. Clin. Endocrinol. Metab. 94, 2332–2337 (2009).
Yamamoto, S. et al. Iatrogenic osteomalacia: report of two cases. J. UOEH 35, 25–31 (2013).
Takeda, Y. et al. Effect of intravenous saccharated ferric oxide on serum FGF23 and mineral metabolism in hemodialysis patients. Am. J. Nephrol. 33, 421–426 (2011).
Shimizu, Y. et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone 45, 814–816 (2009).
Prats, M. et al. Effect of ferric carboxymaltose on serum phosphate and C-terminal FGF23 levels in non-dialysis chronic kidney disease patients: post-hoc analysis of a prospective study. BMC Nephrol. 14, 167 (2013).
Bishay, R. H., Ganda, K. & Seibel, M. J. Long-term iron polymaltose infusions associated with hypophosphataemic osteomalacia: a report of two cases and review of the literature. Ther. Adv. Endocrinol. Metab. 8, 14–19 (2017).
Klein, K., Asaad, S., Econs, M. & Rubin, J. E. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. https://doi.org/10.1136/bcr-2017-222851 (2018).
Huang, L. L. et al. A controlled study of the effects of ferric carboxymaltose on bone and haematinic biomarkers in chronic kidney disease and pregnancy. Nephrol. Dial. Transplant. 33, 1628–1635 (2017).
Silver, J. & Naveh-Many, T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat. Rev. Nephrol. 9, 641–649 (2013).
Gravesen, E., Hofman-Bang, J., Mace, M. L., Lewin, E. & Olgaard, K. High dose intravenous iron, mineral homeostasis and intact FGF23 in normal and uremic rats. BMC Nephrol. 14, 281 (2013).
Larsson, T., Nisbeth, U., Ljunggren, O., Juppner, H. & Jonsson, K. B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 64, 2272–2279 (2003).
Viaene, L. et al. Residual renal function is an independent determinant of serum FGF-23 levels in dialysis patients. Nephrol. Dial. Transplant. 27, 2017–2022 (2012).
Isakova, T. et al. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin. J. Am. Soc. Nephrol. 8, 1009–1018 (2013).
Isakova, T. & Wolf, M. S. FGF23 or PTH: which comes first in CKD? Kidney Int. 78, 947–949 (2010).
Christov, M. et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 84, 776–785 (2013).
Richter, B., Haller, J., Haffner, D. & Leifheit-Nestler, M. Klotho modulates FGF23-mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflugers Arch. 468, 1621–1635 (2016).
Murali, S. K., Andrukhova, O., Clinkenbeard, E. L., White, K. E. & Erben, R. G. Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp mice. PLOS Biol. 14, e1002427 (2016).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made substantial contributions to discussions of the content, and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.E. declares no competing interests. M.W. has served as a consultant to Akebia, AMAG, Amgen, Ardelyx, DiaSorin, Luitpold and Pharmacosmos.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Phosphatonin
-
A collective term for phosphate-regulating factors, including FGF23.
- Vitamin D-resistant hypophosphataemic rickets
-
A class of diseases characterized by low serum phosphate, rickets and resistance to treatment with vitamin D.
- Tumour-induced osteomalacia
-
(TIO). A disease caused by extraosseous FGF23 production by tumour cells, which results in severe hypophosphataemia and osteomalacia.
- Iron economy
-
Regulation of serum iron levels and total body iron stores.
- Autosomal dominant hypophosphatemic rickets
-
(ADHR). An inherited form of hypophosphataemic rickets caused by a mutation in the FGF23 cleavage recognition site, which results in excess FGF23 due to impaired cleavage.
- Absorption
-
The transport of nutrients, including phosphate and calcium, from the intestinal lumen into circulation.
- Tumoural calcinosis
-
A disease caused by a mutation in GALNT3, which results in excess FGF23 cleavage, functional FGF23 deficiency, hyperphosphataemia and diffuse metastatic calcification.
- Autosomal recessive hypophosphataemic rickets
-
(ARHR). An inherited form of hypophosphataemic rickets that can be caused by mutations in the DMP1 gene (type 1) or the ENPP1 gene (type 2), which results in FGF23 excess.
- Acute-phase reactant
-
A circulating factor that increases in response to inflammation.
- Resorption
-
Degradation of bone that releases calcium and phosphate into the circulation.
Rights and permissions
About this article
Cite this article
Edmonston, D., Wolf, M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol 16, 7–19 (2020). https://doi.org/10.1038/s41581-019-0189-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0189-5
This article is cited by
-
The differential effect of modern intravenous iron on fibroblast growth factor 23 and phosphate in non-dialysis dependent CKD – the exploratory randomized controlled double-blind ExplorIRON-CKD study
BMC Nephrology (2024)
-
FGF23 and klotho at the intersection of kidney and cardiovascular disease
Nature Reviews Cardiology (2024)
-
Effect of ferric citrate hydrate on fibroblast growth factor 23 and platelets in non-dialysis-dependent chronic kidney disease and non-chronic kidney disease patients with iron deficiency anemia
Clinical and Experimental Nephrology (2024)
-
FGF-23 is a biomarker of RV dysfunction and congestion in patients with HFrEF
Scientific Reports (2023)
-
Incidence of Hypophosphatemia After Intravenous Administration of Iron: A Matching-Adjusted Indirect Comparison of Data from Japanese Randomized Controlled Trials
Advances in Therapy (2023)