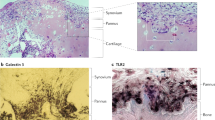
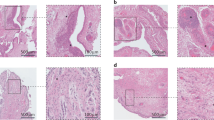
Key Points
-
Synovial tissue is the target tissue for autoimmune arthritides such as rheumatoid arthritis.
-
Synovial biopsy is a safe and well-tolerated procedure that is becoming more widely available.
-
There is a significant body of work from the past 30 years analysing the cellular and molecular changes in synovial tissue from patients with rheumatoid arthritis to identify specific biomarkers.
-
Technological advances in molecular and cellular analysis now provide new opportunities for defining new biomarkers and targets.
Abstract
The synovium is the major target tissue of inflammatory arthritides such as rheumatoid arthritis. The study of synovial tissue has advanced considerably throughout the past few decades from arthroplasty and blind needle biopsy to the use of arthroscopic and ultrasonographic technologies that enable easier visualization and improve the reliability of synovial biopsies. Rapid progress has been made in using synovial tissue to study disease pathogenesis, to stratify patients, to discover biomarkers and novel targets, and to validate therapies, and this progress has been facilitated by increasingly diverse and sophisticated analytical and technological approaches. In this Review, we describe these approaches, and summarize how their use in synovial tissue research has improved our understanding of rheumatoid arthritis and identified candidate biomarkers that could be used in disease diagnosis and stratification, as well as in predicting disease course and treatment response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 December 2017
In the version of this article originally published, the author Sander W. Tas was erroneously omitted from the author list. This error has now been corrected in the online version of the article.
22 September 2017
In the original version of this article the name of one of the authors, Elsa Vieira-Sousa, was incorrectly given as Elsa Sousa. This error has now been corrected in the PDF and HTML versions of the article.
References
Tak, P. P. & Bresnihan, B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 43, 2619–2633 (2000).
Mitchell, D. M. et al. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum. 29, 706–714 (1986).
Pincus, T. et al. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 27, 864–872 (1984).
Wolfe, F., Michaud, K., Gefeller, O. & Choi, H. K. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 48, 1530–1542 (2003).
Sokka, T. et al. Remission and rheumatoid arthritis: data on patients receiving usual care in twenty-four countries. Arthritis Rheum. 58, 2642–2651 (2008).
Balogh, E. et al. Comparison of remission criteria in a tumour necrosis factor inhibitor treated rheumatoid arthritis longitudinal cohort: patient global health is a confounder. Arthritis Res. Ther. 15, R221 (2013).
Fearon, U. & Veale, D. J. Key challenges in rheumatic and musculoskeletal disease translational research. EBioMedicine 1, 95–96 (2014).
Hensvold, A. H. et al. How well do ACPA discriminate and predict RA in the general population: a study based on 12 590 population-representative Swedish twins. Ann. Rheum. Dis. 76, 119–125 (2017).
Rantapaa-Dahlqvist, S. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 48, 2741–2749 (2003).
Choi, I. Y. et al. MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann. Rheum. Dis. 74, 499–505 (2015).
Hueber, W. et al. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res. Ther. 11, R76 (2009).
Smith, M. D. et al. Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann. Rheum. Dis. 62, 303–307 (2003).
Rhee, D. K. et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 115, 622–631 (2005).
Hyc, A., Osiecka-Iwan, A., Niderla-Bielinska, J., Jankowska-Steifer, E. & Moskalewski, S. Pro-and anti-inflammatory cytokines increase hyaluronan production by rat synovial membrane in vitro. Int. J. Mol. Med. 24, 579–585 (2009).
Blewis, M. E. et al. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng. Part A 16, 1329–1337 (2009).
McInnes, I. B. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 (2007).
Tchetverikov, I. et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann. Rheum. Dis. 64, 694–698 (2005).
Billinghurst, R. C. et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest. 99, 1534–1545 (1997).
Shingleton, W. D., Hodges, D. J., Brick, P. & Cawston, T. E. Collagenase: a key enzyme in collagen turnover. Biochem. Cell Biol. 74, 759–775 (1996).
Connolly, M. et al. Acute-phase serum amyloid A regulates tumor necrosis factor α and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 64, 1035–1045 (2011).
Lohmander, L. S., Atley, L. M., Pietka, T. A. & Eyre, D. R. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 48, 3130–3139 (2003).
Tak, P. P. et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 40, 217–225 (1997).
Humby, F. et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 6, e1 (2009).
Vossenaar, E. R. et al. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum. 50, 3485–3494 (2004).
Reece, R. J., Canete, J. D., Parsons, W. J., Emery, P. & Veale, D. J. Distinct vascular patterns of early synovitis in psoriatic, reactive, and rheumatoid arthritis. Arthritis Rheum. 42, 1481–1484 (1999).
Ng, C. T. et al. Synovial tissue hypoxia and inflammation in vivo. Ann. Rheum. Dis. 69, 1389–1395 (2010).
Mullan, R. H. et al. Early changes in serum type II collagen biomarkers predict radiographic progression at one year in inflammatory arthritis patients after biologic therapy. Arthritis Rheum. 56, 2919–2928 (2007).
Månsson, B. et al. Cartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolism. J. Clin. Invest. 95, 1071–1077 (1995).
Biniecka, M. et al. Oxidative damage in synovial tissue is associated with in vivo hypoxic status in the arthritic joint. Ann. Rheum. Dis. 69, 1172–1178 (2010).
Levick, J. R. Permeability of rheumatoid and normal human synovium to specific plasma proteins. Arthritis Rheum. 24, 1550–1560 (1981).
Dahl, L. B., Dahl, I. M., Engström-Laurent, A. & Granath, K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann. Rheum. Dis. 44, 817–822 (1985).
Decker, B., McKenzie, B. F., McGuckin, W. F. & Slocumb, C. H. Comparative distribution of proteins and glycoproteins of serum and synovial fluid. Arthritis Rheum. 2, 162–177 (1959).
Swann, D. A. et al. Role of hyaluronic acid in joint lubrication. Ann. Rheum. Dis. 33, 318–326 (1974).
Hui, A. Y., McCarty, W. J., Masuda, K., Firestein, G. S. & Sah, R. L. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 4, 15–37 (2012).
Rooney, M., Whelan, A., Feighery, C. & Bresnihan, B. Changes in lymphocyte infiltration of the synovial membrane and the clinical course of rheumatoid arthritis. Arthritis Rheum. 32, 361–369 (1989).
Firestein, G. S., Paine, M. M. & Boyle, D. L. Mechanisms of methotrexate action in rheumatoid arthritis. Arthritis Rheum. 37, 193–200 (1994).
Tak, P. P. et al. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 38, 1457–1465 (1995).
Tak, P. P. et al. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor α monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 39, 1077–1081 (1996).
Youssef, P. P. et al. Variability in cytokine and cell adhesion molecule staining in arthroscopic synovial biopsies: quantification using color video image analysis. J. Rheumatol. 24, 2291–2298 (1997).
Kraan, M. C. et al. Modulation of inflammation and metalloproteinase expression in synovial tissue by leflunomide and methotrexate in patients with active rheumatoid arthritis: findings in a prospective, randomized, double-blind, parallel-design clinical trial in thirty-nine. Arthritis Rheum. 43, 1820–1830 (2000).
Gerlag, D. M. et al. Effects of oral prednisolone on biomarkers in synovial tissue and clinical improvement in rheumatoid arthritis. Arthritis Rheum. 50, 3783–3791 (2004).
Cunnane, G., Madigan, A., Murphy, E., FitzGerald, O. & Bresnihan, B. The effects of treatment with interleukin-1 receptor antagonist on the inflamed synovial membrane in rheumatoid arthritis. Rheumatology (Oxford) 40, 62–69 (2001).
Catrina, A. I. et al. Anti-tumour necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology (Oxford) 41, 484–489 (2002).
Smeets, T. J. M., Kraan, M. C., van Loon, M. E. & Tak, P. Tumor necrosis factor α blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum. 48, 2155–2162 (2003).
Kraan, M. C. et al. Differential effects of leflunomide and methotrexate on cytokine production in rheumatoid arthritis. Ann. Rheum. Dis. 63, 1056–1061 (2004).
Rooney, T. et al. Synovial tissue interleukin-18 expression and the response to treatment in patients with inflammatory arthritis. Ann. Rheum. Dis. 63, 1393–1398 (2004).
van Holten, J. et al. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann. Rheum. Dis. 64, 64–69 (2005).
Catrina, A. I. et al. Anti-tumor necrosis factor therapy increases synovial osteoprotegerin expression in rheumatoid arthritis. Arthritis Rheum. 54, 76–81 (2006).
Haringman, J. J. et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 54, 2387–2392 (2006).
Makrygiannakis, D. et al. Intraarticular corticosteroids decrease synovial RANKL expression in inflammatory arthritis. Arthritis Rheum. 54, 1463–1472 (2006).
Gerlag, D. M. & Tak, P. P. Novel approaches for the treatment of rheumatoid arthritis: lessons from the evaluation of synovial biomarkers in clinical trials. Best Pract. Res. Clin. Rheumatol. 22, 311–323 (2008).
Harty, L. C., Gerlag, D. M., Pitzalis, C., Veale, D. J. & Tak, P. P. Synovial tissue analysis for the discovery of diagnostic and prognostic biomarkers in patients with early arthritis. J. Rheumatol. 38, 2068–2072 (2011).
Thurlings, R. M. et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann. Rheum. Dis. 67, 917–925 (2008).
Vergunst, C. E. et al. Modulation of CCR2 in rheumatoid arthritis: a double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 58, 1931–1939 (2008).
van Kuijk, A. W. et al. CCR5 blockade in rheumatoid arthritis: a randomised, double-blind, placebo-controlled clinical trial. Arthritis Rheum. 54, 2387–2392 (2006).
Vergunst, C. E. et al. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 46, 1773–1778 (2007).
Boumans, M. J. et al. Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: a randomised, placebo controlled, proof-of-concept study. Ann. Rheum. Dis. 71, 180–185 (2012).
Gerlag, D. & Tak, P. P. How to perform and analyse synovial biopsies. Best Pract. Res. Clin. Rheumatol. 23, 221–232 (2009).
Kane, D., Veale, D. J., FitzGerald, O. & Reece, R. Survey of arthroscopy performed by rheumatologists. Rheumatology (Oxford) 41, 210–215 (2002).
Lazarou, I. et al. Ultrasound-guided synovial biopsy: a systematic review according to the OMERACT filter and recommendations for minimal reporting standards in clinical studies. Rheumatology (Oxford) 54, 1867–1875 (2015).
Youssef, P. P. et al. Quantitative microscopic analysis of inflammation in rheumatoid arthritis synovial membrane samples selected at arthroscopy compared with samples obtained blindly by needle biopsy. Arthritis Rheum. 41, 663–669 (1998).
Kirkham, B. et al. Intraarticular variability of synovial membrane histology, immunohistology, and cytokine mRNA expression in patients with rheumatoid arthritis. J. Rheumatol. 26, 777–784 (1999).
Smeets, T. J. M. et al. Analysis of the cell infiltrate and expression of matrix metalloproteinases and granzyme B in paired synovial biopsy specimens from the cartilage–pannus junction in patients with RA. Ann. Rheum. Dis. 60, 561–565 (2001).
Soden, M. et al. Immunohistological features in the synovium obtained from clinically uninvolved knee joints of patients with rheumatoid arthritis. Br. J. Rheumatol. 28, 287–292 (1989).
Smith, M. D. et al. Standardisation of synovial tissue infiltrate analysis: how far have we come? How much further do we need to go? Ann. Rheum. Dis. 65, 93–100 (2006).
Mateos, J. et al. Differential protein profiling of synovial fluid from rheumatoid arthritis and osteoarthritis patients using LC–MALDI TOF/TOF. J. Proteomics 75, 2869–2878 (2012).
Biswas, S. et al. Identification of novel autoantigen in the synovial fluid of rheumatoid arthritis patients using an immunoproteomics approach. PLoS ONE 8, e56246 (2013).
Bresnihan, B., Tak, P. P., Emery, P., Klareskog, L. & Breedveld, F. Synovial biopsy in arthritis research: five years of concerted European collaboration. Ann. Rheum. Dis. 59, 506–511 (2000).
Kraan, M. C. et al. Alefacept treatment in psoriatic arthritis: reduction of the effector T cell population in peripheral blood and synovial tissue is associated with improvement of clinical signs of arthritis. Arthritis Rheum. 46, 2776–2784 (2002).
Cañete, J. D. et al. Distinct synovial immunopathology in Behcet disease and psoriatic arthritis. Arthritis Res. Ther. 11, R17 (2009).
van Kuijk, A. W. & Tak, P. P. Synovitis in psoriatic arthritis: immunohistochemistry, comparisons with rheumatoid arthritis, and effects of therapy. Curr. Rheumatol. Rep. 13, 353–359 (2011).
Kobezda, T., Ghassemi-Nejad, S., Mikecz, K., Glant, T. T. & Szekanecz, Z. Of mice and men: how animal models advance our understanding of T-cell function in RA. Nat. Rev. Rheumatol. 10, 160–170 (2014).
Basdeo, S. A. et al. Ex-Th17 (nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J. Immunol. 198, 2249–2259 (2017).
Firestein, G. S. & McInnes, I. B. Immunopathogenesis of rheumatoid arthritis. Immunity 46, 183–196 (2017).
Kraan, M. C. et al. Comparison of synovial tissues from the knee joints and the small joints of rheumatoid arthritis patients: implications for pathogenesis and evaluation of treatment. Arthritis Rheum. 46, 2034–2038 (2002).
Kraan, M. C. et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 41, 1481–1488 (1998).
Ai, R. et al. Joint-specific DNA methylation and transcriptome signatures in rheumatoid arthritis identify distinct pathogenic processes. Nat. Commun. 7, 11849 (2016).
Frank-Bertoncelj, M. et al. Epigenetically-driven anatomical diversity of synovial fibroblasts guides joint-specific fibroblast functions. Nat. Commun. 23, 14852 (2017).
de Hair, M. J. et al. Synovial tissue analysis for the discovery of diagnostic and prognostic biomarkers in patients with early arthritis. J. Rheumatol. 38, 2068–2072 (2011).
van der Heijde, D. M. Joint erosions and patients with early rheumatoid arthritis. Br. J. Rheumatol. 34 (Suppl. 2), 74–78 (1995).
Lard, L. R. et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am. J. Med. 111, 446–451 (2001).
van der Heijde, A. et al. The effectiveness of early treatment with 'second-line' antirheumatic drugs. A randomized, controlled trial. Ann. Intern. Med. 124, 699–707 (1996).
Finckh, A., Liang, M. H., van Herckenrode, C. M. & de Pablo, P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum. 55, 864–872 (2006).
Whiting, P. F. et al. Systematic review: accuracy of anti-citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann. Intern. Med. 152, 456–466 (2010).
Lee, D. M. & Schur, P. H. Clinical utility of the anti-CCP assay in patients with rheumatic diseases. Ann. Rheum. Dis. 62, 870–874 (2003).
Bos, W. H. et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann. Rheum. Dis. 69, 490–494 (2010).
Nielen, M. M. J. et al. Antibodies to citrullinated human fibrinogen (ACF) have diagnostic and prognostic value in early arthritis. Ann. Rheum. Dis. 64, 1199–1204 (2005).
de Hair, M. J. et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheum. 66, 513–522 (2014).
van de Sande, M. G. et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann. Rheum. Dis. 70, 772–777 (2011).
Anderson, A. E. et al. IL-6-driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody-negative rheumatoid arthritis. Ann. Rheum. Dis. 75, 466–473 (2016).
Hensvold, A. H. et al. Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res. Ther. 17, 239 (2015).
Gerlag, D. M., Norris, J. M. & Tak, P. P. Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxford) 55, 607–614 (2016).
Reynisdottir, G. et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann. Rheum. Dis. 75, 1722–1727 (2016).
van Baarsen, L. G. et al. The cellular composition of lymph nodes in the earliest phase of inflammatory arthritis. Ann. Rheum. Dis. 72, 1420–1424 (2013).
Baeten, D. et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann. Rheum. Dis. 59, 945–953 (2000).
Klarenbeek, P. L. et al. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann. Rheum. Dis. 71, 1088–1093 (2012).
Whitaker, J. W. An imprinted rheumatoid arthritis methylome signature reflects pathogenic phenotype. Genome Med. 5, 40 (2013).
Yeo, L. et al. Expression of chemokines CXCL4 and CXCL7 by synovial macrophages defines an early stage of rheumatoid arthritis. Ann. Rheum. Dis. 75, 763–771 (2016).
Kraan, M. C. et al. Immunohistological analysis of synovial tissue for differential diagnosis in early arthritis. Rheumatology (Oxford) 38, 1074–1080 (1999).
van de Sande, M. G. et al. Local synovial engagement of angiogenic TIE-2 is associated with the development of persistent erosive rheumatoid arthritis in patients with early arthritis. Arthritis Rheum. 65, 3073–3083 (2013).
de Launay, D. et al. Selective involvement of ERK and JNK mitogen-activated protein kinases in early rheumatoid arthritis (1987 ACR criteria compared to 2010 ACR/EULAR criteria): a prospective study aimed at identification of diagnostic and prognostic biomarkers as well as therapeutic targets. Ann. Rheum. Dis. 71, 415–423 (2012).
Drossaers-Bakker, K. W. et al. Long-term outcome in rheumatoid arthritis: a simple algorithm of baseline parameters can predict radiographic damage, disability, and disease course at 12-year followup. Arthritis Rheum. 47, 383–390 (2002).
Scott, D. L. The diagnosis and prognosis of early arthritis: rationale for new prognostic criteria. Arthritis Rheum. 46, 286–290 (2002).
Lindstrom, T. M. & Robinson, W. H. Biomarkers for rheumatoid arthritis: making it personal. Scand. J. Clin. Lab. Investig. 70, 79–84 (2010).
Trusheim, M. R., Berndt, E. R. & Douglas, F. L. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat. Rev. Drug Discov. 6, 287–293 (2007).
Edwards, J. C. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
Genovese, M. C. et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N. Engl. J. Med. 353, 1114–1123 (2005).
Moreland, L. W. et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N. Engl. J. Med. 337, 141–147 (1997).
Pitzalis, C. et al. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 14, 447–462 (2014).
Thurlings, R. M. et al. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum. 58, 1582–1589 (2008).
Cantaert, T. et al. B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J. Immunol. 181, 785–794 (2008).
Klaasen, R. et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum. 60, 3217–3224 (2009).
van de Sande, M. G. et al. Presence of lymphocyte aggregates in the synovium of patients with early arthritis in relationship to diagnosis and outcome: is it a constant feature over time? Ann. Rheum. Dis. 70, 700–703 (2011).
Lindberg, J. et al. The gene expression profile in the synovium as a predictor of the clinical response to infliximab treatment in rheumatoid arthritis. PLoS ONE 5, e11310 (2010).
Dolhain, R. J. et al. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Rheumatology (Oxford) 37, 502–508 (1998).
Walsh, C. A. E., Fearon, U., FitzGerald, O., Veale, D. J. & Bresnihan, B. Decreased CD20 expression in rheumatoid arthritis synovium following 8 weeks of rituximab therapy. Clin. Exp. Rheumatol. 26, 656 (2008).
Vos, K. et al. Early effects of rituximab on the synovial cell infiltrate in patients with rheumatoid arthritis. Arthritis Rheum. 56, 772–778 (2007).
Bresnihan, B. et al. Synovial macrophages as a biomarker of response to therapeutic intervention in rheumatoid arthritis: standardization and consistency across centers. J. Rheumatol. 36, 1800–1802 (2009).
Kavanaugh, A. et al. Assessment of rituximab's immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann. Rheum. Dis. 67, 402–408 (2008).
Yanni, G., Nabil, M., Farahat, M. R., Poston, R. N. & Panayi, G. S. Intramuscular gold decreases cytokine expression and macrophage numbers in the rheumatoid synovial membrane. Ann. Rheum. Dis. 53, 315–322 (1994).
Smith, M. D. et al. Treatment-induced remission in rheumatoid arthritis patients is characterized by a reduction in macrophage content of synovial biopsies. Rheumatology (Oxford) 40, 367–374 (2001).
Wijbrandts, C. A. et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor α expression in the synovium. Ann. Rheum. Dis. 67, 1139–1144 (2008).
Haringman, J. J. et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 64, 834–838 (2005).
Boumans, M. J. et al. Response to rituximab in patients with rheumatoid arthritis in different compartments of the immune system. Arthritis Rheum. 63, 3187–3194 (2011).
Wijbrandts, C. A. et al. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 56, 3869–3871 (2007).
Bresnihan, B. et al. Synovial tissue analysis in clinical trials. J. Rheumatol. 32, 2481–2484 (2005).
Buch, M. H. et al. Mode of action of abatacept in rheumatoid arthritis patients having failed tumour necrosis factor blockade: a histological, gene expression and dynamic magnetic resonance imaging pilot study. Ann. Rheum. Dis. 68, 1220–1227 (2009).
Boyle, D. L. et al. The JAK inhibitor tofacitinib suppresses synovial JAK1–STAT signalling in rheumatoid arthritis. Ann. Rheum. Dis. 74, 1311–1316 (2015).
Haringman, J. J., Kraan, M. C., Smeets, T. J., Zwinderman, K. H. & Tak, P. P. Chemokine blockade and chronic inflammatory disease: proof of concept in patients with rheumatoid arthritis. Ann. Rheum. Dis. 62, 715–721 (2003).
Tak, P. P. et al. Chemokine receptor CCR1 antagonist CCX354-C treatment for rheumatoid arthritis: CARAT-2, a randomised, placebo controlled clinical trial. Ann. Rheum. Dis. 72, 337–344 (2012).
Mullan, R. H. et al. Acute-phase serum amyloid A stimulation of angiogenesis, leukocyte recruitment, and matrix degradation in rheumatoid arthritis through an NF-κB-dependent signal transduction pathway. Arthritis Rheum. 54, 105–114 (2006).
Connolly, M. et al. Acute serum amyloid A is an endogenous TLR2 ligand that mediates inflammatory and angiogenic mechanisms. Ann. Rheum. Dis. 75, 1392–1398 (2016).
Connolly, M., Veale, D. J. & Fearon, U. Acute serum amyloid A regulates cytoskeletal rearrangement, cell matrix interactions and promotes cell migration in rheumatoid arthritis. Ann. Rheum. Dis. 70, 1296–1303 (2011).
Haringman, J. J., Ludikhuize, J. & Tak, P. P. Chemokines in joint disease: the key to inflammation? Ann. Rheum. Dis. 63, 1186–1194 (2004).
Kraan, M. C. et al. The development of clinical signs of rheumatoid synovial inflammation is associated with increased synthesis of the chemokine CXCL8 (interleukin-8). Arthritis Res. 3, 65–71 (2001).
Kang, K. Y. et al. S100A8/A9 as a biomarker for synovial inflammation and joint damage in patients. Kor. J. Intern. Med. 29, 12–19 (2014).
Wittkowski, H. et al. MRP8 and MRP14, phagocyte-specific danger signals, are sensitive biomarkers of disease activity in cryopyrin-associated periodic syndromes. Ann. Rheum. Dis. 70, 2075–2081 (2011).
Meijer, B., Gearry, R. B. & Day, A. S. The role of S100A12 as a systemic marker of inflammation. Int. J. Inflam 2012, 907078 (2012).
Liao, H. et al. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. 50, 3792–3803 (2004).
Green, M. J. et al. Serum MMP-3 and MMP-1 and progression of joint damage in early rheumatoid arthritis. Rheumatology (Oxford) 42, 83–88 (2003).
Ishiguro, N. et al. Relationships of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover and inflammation as revealed by analyses of synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 44, 2503–2511 (2001).
Chandran, V. Soluble biomarkers may differentiate psoriasis from psoriatic arthritis. J. Rheumatol. 89, 65–66 (2012).
Boumans, M. J. et al. Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann. Rheum. Dis. 71, 108–113 (2012).
Masson-Bessiere, C. et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the α- and β-chains of fibrin. J. Immunol. 166, 4177–4184 (2001).
Després, N., Boire, G., Lopez-Longo, F. J. & Ménard, H. A. The Sa system: a novel antigen-antibody system specific for rheumatoid arthritis. J. Rheumatol. 21, 1027–1033 (1994).
Baeten, D. et al. Specific presence of intracellular citrullinated proteins in rheumatoid arthritis synovium: relevance to antifilaggrin autoantibodies. Arthritis Rheum. 44, 2255–2262 (2001).
Masson-Bessiere, C. et al. In the rheumatoid pannus, anti-filaggrin autoantibodies are produced by local plasma cells and constitute a higher proportion of IgG than in synovial fluid and serum. Clin. Exp. Immunol. 119, 544–552 (2000).
Woetzel, D. et al. Identification of rheumatoid arthritis and osteoarthritis patients by transcriptome-based rule set generation. Arthritis Res. Ther. 16, R84 (2014).
Huber, R. et al. Identification of intra-group, inter-individual, and gene-specific variances in mRNA expression profiles in the rheumatoid arthritis synovial membrane. Arthritis Res. Ther. 10, R98 (2008).
van der Pouw Kraan, T. C. et al. Responsiveness to anti-tumour necrosis factor α therapy is related to pre-treatment tissue inflammation levels in rheumatoid arthritis patients. Ann. Rheum. Dis. 67, 563–566 (2008).
Badot, V. et al. Gene expression profiling in the synovium identifies a predictive signature of absence of response to adalimumab therapy in rheumatoid arthritis. Arthritis Res. Ther. 11, R57 (2009).
Gutierrez-Roelens, I. et al. Rituximab treatment induces the expression of genes involved in healing processes in the rheumatoid arthritis synovium. Arthritis Rheum. 63, 1246–1254 (2011).
Colburn, W. A. Selecting and validating biologic markers for drug development. J. Clin. Pharmacol. 37, 355–362 (1997).
van de Sande, M. G. H. et al. Evaluating antirheumatic treatments using synovial biopsy: a recommendation for standardisation to be used in clinical trials. Ann. Rheum. Dis. 70, 423–427 (2011).
Choi, I. Y., Gerlag, D. M., Holzinger, D., Roth, J. & Tak, P. P. From synovial tissue to peripheral blood: myeloid related protein 8/14 is a sensitive biomarker for effective treatment in early drug development in patients with rheumatoid arthritis. PLoS ONE 9, e106253 (2014).
Dennis, G. Jr et al. Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res. Ther. 16, R90 (2014).
Tan, P. K. et al. Evaluation of gene expression measurements from commercial microarray platforms. Nucleic Acids Res. 31, 5676–5684 (2003).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Gao, W. et al. Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2. Ann. Rheum. Dis. 72, 1080–1088 (2013).
Ebhardt, H. A. et al. Applications of targeted proteomics in systems biology and translational medicine. Proteomics 15, 3193–3208 (2015).
Smith, S. L., Plant, D., Eyre, S. & Barton, A. The potential use of expression profiling: implications for predicting treatment response in rheumatoid arthritis. Ann. Rheum. Dis. 72, 1118–1124 (2013).
Häupl, T., Stuhlmüller, B., Grützkau, A., Radbruch, A. & Burmester, G. R. Does gene expression analysis inform us in rheumatoid arthritis? Ann. Rheum. Dis. 69, i37–i42 (2010).
van Baarsen, L. G. M. et al. Synovial tissue heterogeneity in rheumatoid arthritis in relation to disease activity and biomarkers in peripheral blood. Arthritis Rheum. 62, 1602–1607 (2010).
Acknowledgements
We wish to thank all our colleagues in the European Synovitis Study Group and in the OMERACT group who have supported the development of synovial tissue research.
Author information
Authors and Affiliations
Contributions
D.J.V., C.O., U.F., A.N., A.P., S.W.T. and J.E.F. researched data for the article, made a substantial contribution to the discussion of article content, wrote the manuscript, and reviewed and edited the manuscript before submission. E.V.-S., F.H., S.A.J., T. McG. and R.T. researched data for the article, wrote the manuscript, and reviewed and edited the manuscript before submission. A.F. researched data for the article, and reviewed and edited the manuscript before submission. B.R.L. wrote the manuscript, and reviewed and edited the manuscript before submission. D.L.B., M.H.B., C.D.B., J.D.C., A.I.C., E.H.C., P.E., D.G., J.D.I., B.L., A.M., I.B.M., C.P., M.S. and P.P.T. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Fibroblast-like synoviocytes
-
(FLSs). Also known as type B synovial lining cells, FLSs account for the majority of cells in the synovial lining layer.
- Intimal lining layer
-
The lining of the synovium comprising a few cells without a basement membrane and which covers the nonarticular surface of the joint capsule.
- Synovial sublining
-
A loose connective tissue that lies beneath the intimal lining of the synovium.
- Pannus
-
A 'tumour-like' mass of hyperplastic synovial tissue that expands into the joint, invading into bone and cartilage.
- Arthroplasty
-
Surgical reconstruction or replacement of a synovial joint.
- Arthroscopic biopsy
-
Minimally invasive procedure to examine a synovial joint using an endoscope.
- Ex-TH17 cells
-
T helper 17 (TH17) cells can switch to become ex-TH17 cells that no longer produce IL-17 but have the ability to produce IFNγ.
- Positional memory
-
Cells might demonstrate different DNA 'fingerprints' depending on the site of the body at which they reside.
- Undifferentiated arthritis
-
Inflammatory oligoarthritis or polyarthritis that does not conform to any of the recognized inflammatory arthritis types.
- Disease stratification
-
The concept that a disease can be classified into distinct subsets that exhibit differential outcomes and responses, and that can each be labelled by a biomarker or a combination of biomarkers.
Rights and permissions
About this article
Cite this article
Orr, C., Vieira-Sousa, E., Boyle, D. et al. Synovial tissue research: a state-of-the-art review. Nat Rev Rheumatol 13, 463–475 (2017). https://doi.org/10.1038/nrrheum.2017.115
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.115
This article is cited by
-
A landscape of gene expression regulation for synovium in arthritis
Nature Communications (2024)
-
Profiling joint tissues at single-cell resolution: advances and insights
Nature Reviews Rheumatology (2024)
-
Definition of follicular helper T cell and cytokines expression in synovial fluid of rheumatoid arthritis
Clinical Rheumatology (2024)
-
The access route through the anatomical snuffbox in ultrasound-guided synovial biopsy of the wrist allows for a safe and effective collection of tissue samples in inflammatory arthritis
Arthritis Research & Therapy (2023)
-
SMAD2 inhibits pyroptosis of fibroblast-like synoviocytes and secretion of inflammatory factors via the TGF-β pathway in rheumatoid arthritis
Arthritis Research & Therapy (2023)