Key Points
-
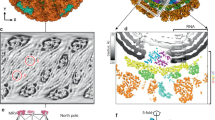
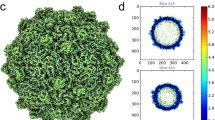
Several high-resolution structures of rotavirus proteins and particles have recently been reported. These structures have substantially expanded our understanding of cell entry, genome replication and virion assembly for rotaviruses.
-
The rotavirus outer capsid functions as a sensor to effect membrane penetration and allow the viruses to gain access to the host cell cytosol. Structures of the major outer capsid protein suggest a model for triggered disassembly in endosomal vesicles.
-
Despite the fact that rotaviruses are non-enveloped viruses, their method of membrane penetration resembles that of membrane fusion for enveloped viruses. Structures of the outer-capsid spike protein in pre- and post-membrane penetration conformations suggest a working model.
-
Rotaviruses, like other members of the family Reoviridae, direct RNA transcription from inside a subviral particle that never disassembles in the viral life cycle. This process is presumably triggered by the loss of the outer capsid during entry.
-
The assembly of subviral particles occurs in non-membranous cytoplasmic inclusions and is likely to be regulated by several viral non-structural proteins. Assembly of the rotavirus core shell around complexes containing viral positive-sense RNA and RNA polymerase induces conformational changes that trigger synthesis of the double-stranded RNA genome.
-
Assembly of the rotavirus outer capsid involves the budding of a subviral particle through the endoplasmic reticulum membrane. Subsequent removal of the membrane from the viral particle is concomitant with the addition of outer capsid proteins. The process of membrane acquisition and removal during assembly is one of the least understood aspects of rotavirus biology.
-
The process by which rotaviruses are released from infected cells is also poorly characterized. The mechanism of viral egress is probably cell type specific and may differ in vitro versus in vivo.
-
Assembly-mediated regulation of replication is not specific to rotaviruses or to the Reoviridae family. Other viruses, including hepatitis B virus, have been shown to use assembly state intermediates as both checkpoints and triggers to ensure that replication occurs in a controlled and productive manner.
Abstract
Viral replication is rapid and robust, but it is far from a chaotic process. Instead, successful production of infectious progeny requires that events occur in the correct place and at the correct time. Rotaviruses (segmented double-stranded RNA viruses of the Reoviridae family) seem to govern their replication through ordered disassembly and assembly of a triple-layered icosahedral capsid. In recent years, high-resolution structural data have provided unprecedented insight into these events. In this Review, we explore the current understanding of rotavirus replication and how it compares to replication of other Reoviridae family members.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
09 December 2013
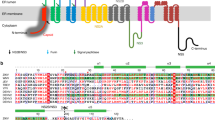
Figure 2 in the above article is based on a copyrighted figure by SIB Swiss Institute of Bioinformatics made available at the following website: http://viralzone.expasy.org/complete_by_species/107.html. The figure was modified with permission and rights to the modified figure are retained by the SIB Swiss Institute of Bioinformatics. The figure legend has been corrected online.
References
Settembre, E. C., Chen, J. Z., Dormitzer, P. R., Grigorieff, N. & Harrison, S. C. Atomic model of an infectious rotavirus particle. EMBO J. 30, 408–416 (2011). This work determines a high-resolution structure for the entire rotavirus virion, including the unexpected conformation of the primed spike.
Ludert, J. E., Ruiz, M. C., Hidalgo, C. & Liprandi, F. Antibodies to rotavirus outer capsid glycoprotein VP7 neutralize infectivity by inhibiting virion decapsidation. J. Virol. 76, 6643–6651 (2002).
Chen, J. Z. et al. Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM. Proc. Natl Acad. Sci. USA 106, 10644–10648 (2009). This report describes the unusual 'grip arm' mode of VP7 assembly onto the rotavirus subviral particle.
Lawton, J. A., Estes, M. K. & Prasad, B. V. Three-dimensional visualization of mRNA release from actively transcribing rotavirus particles. Nature Struct. Biol. 4, 118–121 (1997).
Chen, D. & Patton, J. T. De novo synthesis of minus strand RNA by the rotavirus RNA polymerase in a cell-free system involves a novel mechanism of initiation. RNA 6, 1455–1467 (2000).
McDonald, S. M. & Patton, J. T. Rotavirus VP2 core shell regions critical for viral polymerase activation. J. Virol. 85, 3095–3105 (2011). This investigation identifies the sites of interaction between the viral RNA polymerase and the VP2 decamer that are necessary for triggering RNA synthesis.
Aoki, S. T. et al. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science 324, 1444–1447 (2009).
Dormitzer, P. R., Nason, E. B., Prasad, B. V. & Harrison, S. C. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature 430, 1053–1058 (2004). This article proposes the current model for membrane penetration by rotaviruses.
Dormitzer, P. R., Sun, Z. Y., Wagner, G. & Harrison, S. C. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21, 885–897 (2002).
Lu, X. et al. Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1. Structure 16, 1678–1688 (2008). This report provides a mechanistic explanation for VP1 autoinhibition and suggests several conformational changes that may be required for polymerase activity.
McClain, B., Settembre, E., Temple, B. R., Bellamy, A. R. & Harrison, S. C. X-ray crystal structure of the rotavirus inner capsid particle at 3.8 Å resolution. J. Mol. Biol. 397, 587–599 (2010).
Zhang, X. et al. Near-atomic resolution using electron cryomicroscopy and single-particle reconstruction. Proc. Natl Acad. Sci. USA 105, 1867–1872 (2008).
Reinisch, K. M., Nibert, M. L. & Harrison, S. C. Structure of the reovirus core at 3.6 Å resolution. Nature 404, 960–967 (2000).
Grimes, J. M. et al. The atomic structure of the bluetongue virus core. Nature 395, 470–478 (1998).
Zhang, X., Walker, S. B., Chipman, P. R., Nibert, M. L. & Baker, T. S. Reovirus polymerase λ3 localized by cryo-electron microscopy of virions at a resolution of 7.6 Å. Nature Struct. Biol. 10, 1011–1018 (2003).
Estrozi, L. F. & Navaza, J. Ab initio high-resolution single-particle 3D reconstructions: the symmetry adapted functions way. J. Struct. Biol. 172, 253–260 (2010).
Mathieu, M. et al. Atomic structure of the major capsid protein of rotavirus: implications for the architecture of the virion. EMBO J. 20, 1485–1497 (2001).
Matthijnssens, J. et al. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 153, 1621–1629 (2008).
Zhang, X. et al. Features of reovirus outer capsid protein μ1 revealed by electron cryomicroscopy and image reconstruction of the virion at 7.0 Å resolution. Structure 13, 1545–1557 (2005).
Liemann, S., Chandran, K., Baker, T. S., Nibert, M. L. & Harrison, S. C. Structure of the reovirus membrane-penetration protein, μ1, in a complex with is protector protein, σ3. Cell 108, 283–295 (2002).
Estes, M. K., Graham, D. Y. & Mason, B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J. Virol. 39, 879–888 (1981).
Crawford, S. E. et al. Trypsin cleavage stabilizes the rotavirus VP4 spike. J. Virol. 75, 6052–6061 (2001).
Yeager, M., Berriman, J. A., Baker, T. S. & Bellamy, A. R. Three-dimensional structure of the rotavirus haemagglutinin VP4 by cryo-electron microscopy and difference map analysis. EMBO J. 13, 1011–1018 (1994).
Li, Z., Baker, M. L., Jiang, W., Estes, M. K. & Prasad, B. V. Rotavirus architecture at subnanometer resolution. J. Virol. 83, 1754–1766 (2009).
Shaw, A. L. et al. Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell 74, 693–701 (1993).
Fiore, L., Greenberg, H. B. & Mackow, E. R. The VP8 fragment of VP4 is the rhesus rotavirus hemagglutinin. Virology 181, 553–563 (1991).
Ruggeri, F. M. & Greenberg, H. B. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J. Virol. 65, 2211–2219 (1991).
Monnier, N. et al. High-resolution molecular and antigen structure of the VP8* core of a sialic acid-independent human rotavirus strain. J. Virol. 80, 1513–1523 (2006).
Dormitzer, P. R. et al. Specificity and affinity of sialic acid binding by the rhesus rotavirus VP8* core. J. Virol. 76, 10512–10517 (2002).
Blanchard, H., Yu, X., Coulson, B. S. & von Itzstein, M. Insight into host cell carbohydrate-recognition by human and porcine rotavirus from crystal structures of the virion spike associated carbohydrate-binding domain (VP8*). J. Mol. Biol. 367, 1215–1226 (2007).
Mendez, E., Arias, C. F. & Lopez, S. Binding to sialic acids is not an essential step for the entry of animal rotaviruses to epithelial cells in culture. J. Virol. 67, 5253–5259 (1993).
Ciarlet, M. & Estes, M. K. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity. J. Gen. Virol. 80, 943–948 (1999).
Kim, I. S., Trask, S. D., Babyonyshev, M., Dormitzer, P. R. & Harrison, S. C. Effect of mutations in VP5 hydrophobic loops on rotavirus cell entry. J. Virol. 84, 6200–6207 (2010).
Tihova, M., Dryden, K. A., Bellamy, A. R., Greenberg, H. B. & Yeager, M. Localization of membrane permeabilization and receptor binding sites on the VP4 hemagglutinin of rotavirus: implications for cell entry. J. Mol. Biol. 314, 985–992 (2001).
Dormitzer, P. R., Greenberg, H. B. & Harrison, S. C. Proteolysis of monomeric recombinant rotavirus VP4 yields an oligomeric VP5* core. J. Virol. 75, 7339–7350 (2001).
Harrison, S. C. Viral membrane fusion. Nature Struct. Mol. Biol. 15, 690–698 (2008).
Wolf, M., Vo, P. T. & Greenberg, H. B. Rhesus rotavirus entry into a polarized epithelium is endocytosis dependent and involves sequential VP4 conformational changes. J. Virol. 85, 2492–2503 (2011). This evaluation of the rotavirus entry process identifies several cellular requirements and provides support for predicted conformational changes in the virion.
Trask, S. D., Kim, I. S., Harrison, S. C. & Dormitzer, P. R. A rotavirus spike protein conformational intermediate binds lipid bilayers. J. Virol. 84, 1764–1770 (2010).
Agosto, M. A., Ivanovic, T. & Nibert, M. L. Mammalian reovirus, a nonfusogenic nonenveloped virus, forms size-selective pores in a model membrane. Proc. Natl Acad. Sci. USA 103, 16496–16501 (2006).
Zhang, L. et al. Requirements for the formation of membrane pores by the reovirus myristoylated micro1N peptide. J. Virol. 83, 7004–7014 (2009).
Arias, C. F., Romero, P., Alvarez, V. & Lopez, S. Trypsin activation pathway of rotavirus infectivity. J. Virol. 70, 5832–5839 (1996).
Chemello, M. E., Aristimuno, O. C., Michelangeli, F. & Ruiz, M. C. Requirement for vacuolar H+-ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells. J. Virol. 76, 13083–13087 (2002).
Ludert, J. E., Michelangeli, F., Gil, F., Liprandi, F. & Esparza, J. Penetration and uncoating of rotaviruses in cultured cells. Intervirology 27, 95–101 (1987).
Yoder, J. D. et al. VP5* rearranges when rotavirus uncoats. J. Virol. 83, 11372–11377 (2009).
Ebert, D. H., Deussing, J., Peters, C. & Dermody, T. S. Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277, 24609–24617 (2002).
Golden, J. W., Bahe, J. A., Lucas, W. T., Nibert, M. L. & Schiff, L. A. Cathepsin S. supports acid-independent infection by some reoviruses. J. Biol. Chem. 279, 8547–8557 (2004).
Lawton, J. A., Estes, M. K. & Prasad, B. V. Mechanism of genome transcription in segmented dsRNA viruses. Adv. Virus Res. 55, 185–229 (2000).
Tao, Y., Farsetta, D. L., Nibert, M. L. & Harrison, S. C. RNA synthesis in a cage—structural studies of reovirus polymerase λ3. Cell 111, 733–745 (2002). This is the first study to report an atomic structure of an RNA polymerase for a member of the Reoviridae , revealing the four-tunnel architecture of the enzyme.
Chen, D., Luongo, C. L., Nibert, M. L. & Patton, J. T. Rotavirus open cores catalyze 5′-capping and methylation of exogenous RNA: evidence that VP3 is a methyltransferase. Virology 265, 120–130 (1999).
Sutton, G., Grimes, J. M., Stuart, D. I. & Roy, P. Bluetongue virus VP4 is an RNA-capping assembly line. Nature Struct. Mol. Biol. 14, 449–451 (2007). This paper reports the atomic structure of the bluetongue virus RNA-capping enzyme, the only capping enzyme of a non-turreted Reoviridae virus that has been described to date.
Kim, J., Parker, J. S., Murray, K. E. & Nibert, M. L. Nucleoside and RNA triphosphatase activities of orthoreovirus transcriptase cofactor μ2. J. Biol. Chem. 279, 4394–4403 (2004).
Cheng, L. et al. Atomic model of a cypovirus built from cryo-EM structure provides insight into the mechanism of mRNA capping. Proc. Natl Acad. Sci. USA 108, 1373–1378 (2011).
Mendez, I. I., Weiner, S. G., She, Y. M., Yeager, M. & Coombs, K. M. Conformational changes accompany activation of reovirus RNA-dependent RNA transcription. J. Struct. Biol. 162, 277–289 (2008).
Libersou, S. et al. Geometric mismatches within the concentric layers of rotavirus particles: a potential regulatory switch of viral particle transcription activity. J. Virol. 82, 2844–2852 (2008).
Thouvenin, E. et al. Antibody inhibition of the transcriptase activity of the rotavirus DLP: a structural view. J. Mol. Biol. 307, 161–172 (2001).
Feng, N. et al. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA mAb. J. Clin. Invest. 109, 1203–1213 (2002).
Mansell, E. A. & Patton, J. T. Rotavirus RNA replication: VP2, but not VP6, is necessary for viral replicase activity. J. Virol. 64, 4988–4996 (1990).
Patton, J. T. Rotavirus VP1 alone specifically binds to the 3′ end of viral mRNA, but the interaction is not sufficient to initiate minus-strand synthesis. J. Virol. 70, 7940–7947 (1996).
Patton, J. T., Jones, M. T., Kalbach, A. N., He, Y. W. & Xiaobo, J. Rotavirus RNA polymerase requires the core shell protein to synthesize the double-stranded RNA genome. J. Virol. 71, 9618–9626 (1997).
McDonald, S. M. & Patton, J. T. Molecular characterization of a subgroup specificity associated with the rotavirus inner capsid protein VP2. J. Virol. 82, 2752–2764 (2008).
Silvestri, L. S., Taraporewala, Z. F. & Patton, J. T. Rotavirus replication: plus-sense templates for double-stranded RNA synthesis are made in viroplasms. J. Virol. 78, 7763–7774 (2004).
Jayaram, H., Taraporewala, Z., Patton, J. T. & Prasad, B. V. Rotavirus protein involved in genome replication and packaging exhibits a HIT-like fold. Nature 417, 311–315 (2002).
Jiang, X. et al. Cryoelectron microscopy structures of rotavirus NSP2-NSP5 and NSP2-RNA complexes: implications for genome replication. J. Virol. 80, 10829–10835 (2006).
Martin, D., Ouldali, M., Menetrey, J. & Poncet, D. Structural organisation of the rotavirus nonstructural protein NSP5. J. Mol. Biol. 413, 209–221 (2011).
Chnaiderman, J., Barro, M. & Spencer, E. NSP5 phosphorylation regulates the fate of viral mRNA in rotavirus infected cells. Arch. Virol. 147, 1899–1911 (2002).
Ogden, K. M., Ramanathan, H. N. & Patton, J. T. Residues of the rotavirus RNA-dependent RNA polymerase template entry tunnel that mediate RNA recognition and genome replication. J. Virol. 85, 1958–1969 (2011).
Noda, T. et al. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439, 490–492 (2006).
Gao, Q. & Palese, P. Rewiring the RNAs of influenza virus to prevent reassortment. Proc. Natl Acad. Sci. USA 106, 15891–15896 (2009).
Kobayashi, T. et al. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 1, 147–157 (2007).
Roner, M. R. & Joklik, W. K. Reovirus reverse genetics: incorporation of the CAT gene into the reovirus genome. Proc. Natl Acad. Sci. USA 98, 8036–8041 (2001).
Li, W. et al. Genomic analysis of codon, sequence and structural conservation with selective biochemical-structure mapping reveals highly conserved and dynamic structures in rotavirus RNAs with potential cis-acting functions. Nucleic Acids Res. 38, 7718–7735 (2010).
Patton, J. T., Wentz, M., Xiaobo, J. & Ramig, R. F. cis-acting signals that promote genome replication in rotavirus mRNA. J. Virol. 70, 3961–3971 (1996).
Lepault, J. et al. Structural polymorphism of the major capsid protein of rotavirus. EMBO J. 20, 1498–1507 (2001).
Zeng, C. Q. et al. Characterization of rotavirus VP2 particles. Virology 201, 55–65 (1994).
Berois, M., Sapin, C., Erk, I., Poncet, D. & Cohen, J. Rotavirus nonstructural protein NSP5 interacts with major core protein VP2. J. Virol. 77, 1757–1763 (2003).
Kattoura, M. D., Chen, X. & Patton, J. T. The rotavirus RNA-binding protein NS35 (NSP2) forms 10S multimers and interacts with the viral RNA polymerase. Virology 202, 803–813 (1994).
Vende, P., Tortorici, M. A., Taraporewala, Z. F. & Patton, J. T. Rotavirus NSP2 interferes with the core lattice protein VP2 in initiation of minus-strand synthesis. Virology 313, 261–273 (2003).
Bergmann, C. C., Maass, D., Poruchynsky, M. S., Atkinson, P. H. & Bellamy, A. R. Topology of the non-structural rotavirus receptor glycoprotein NS28 in the rough endoplasmic reticulum. EMBO J. 8, 1695–1703 (1989).
Bowman, G. D. et al. Crystal structure of the oligomerization domain of NSP4 from rotavirus reveals a core metal-binding site. J. Mol. Biol. 304, 861–871 (2000).
O'Brien, J. A., Taylor, J. A. & Bellamy, A. R. Probing the structure of rotavirus NSP4: a short sequence at the extreme C terminus mediates binding to the inner capsid particle. J. Virol. 74, 5388–5394 (2000).
Taylor, J. A., O'Brien, J. A. & Yeager, M. The cytoplasmic tail of NSP4, the endoplasmic reticulum-localized non-structural glycoprotein of rotavirus, contains distinct virus binding and coiled coil domains. EMBO J. 15, 4469–4476 (1996).
Taylor, J. A., O'Brien, J. A., Lord, V. J., Meyer, J. C. & Bellamy, A. R. The RER-localized rotavirus intracellular receptor: a truncated purified soluble form is multivalent and binds virus particles. Virology 194, 807–814 (1993).
Au, K. S., Mattion, N. M. & Estes, M. K. A subviral particle binding domain on the rotavirus nonstructural glycoprotein NS28. Virology 194, 665–673 (1993).
Trask, S. D. & Dormitzer, P. R. Assembly of highly infectious rotavirus particles recoated with recombinant outer capsid proteins. J. Virol. 80, 11293–11304 (2006).
Poruchynsky, M. S. & Atkinson, P. H. Rotavirus protein rearrangements in purified membrane-enveloped intermediate particles. J. Virol. 65, 4720–4727 (1991).
Maass, D. R. & Atkinson, P. H. Rotavirus proteins VP7, NS28, and VP4 form oligomeric structures. J. Virol. 64, 2632–2641 (1990).
Stirzaker, S. C. & Both, G. W. The signal peptide of the rotavirus glycoprotein VP7 is essential for its retention in the ER as an integral membrane protein. Cell 56, 741–747 (1989).
Petrie, B. L., Greenberg, H. B., Graham, D. Y. & Estes, M. K. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1, 133–152 (1984).
Gonzalez, R. A., Espinosa, R., Romero, P., Lopez, S. & Arias, C. F. Relative localization of viroplasmic and endoplasmic reticulum-resident rotavirus proteins in infected cells. Arch. Virol. 145, 1963–1973 (2000).
Poruchynsky, M. S., Maass, D. R. & Atkinson, P. H. Calcium depletion blocks the maturation of rotavirus by altering the oligomerization of virus-encoded proteins in the ER. J. Cell Biol. 114, 651–656 (1991).
Michelangeli, F., Liprandi, F., Chemello, M. E., Ciarlet, M. & Ruiz, M. C. Selective depletion of stored calcium by thapsigargin blocks rotavirus maturation but not the cytopathic effect. J. Virol. 69, 3838–3847 (1995).
Browne, E. P., Bellamy, A. R. & Taylor, J. A. Membrane-destabilizing activity of rotavirus NSP4 is mediated by a membrane-proximal amphipathic domain. J. Gen. Virol. 81, 1955–1959 (2000).
Charpilienne, A. et al. Solubilized and cleaved VP7, the outer glycoprotein of rotavirus, induces permeabilization of cell membrane vesicles. J. Gen. Virol. 78, 1367–1371 (1997).
Silvestri, L. S., Tortorici, M. A., Vasquez-Del Carpio, R. & Patton, J. T. Rotavirus glycoprotein NSP4 is a modulator of viral transcription in the infected cell. J. Virol. 79, 15165–15174 (2005).
Lopez, T. et al. Silencing the morphogenesis of rotavirus. J. Virol. 79, 184–192 (2005).
Musalem, C. & Espejo, R. T. Release of progeny virus from cells infected with simian rotavirus SA11. J. Gen. Virol. 66, 2715–2724 (1985).
Jourdan, N. et al. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J. Virol. 71, 8268–8278 (1997).
Cudmore, S., Cossart, P., Griffiths, G. & Way, M. Actin-based motility of vaccinia virus. Nature 378, 636–638 (1995).
Boyce, M., Celma, C. C. & Roy, P. Development of reverse genetics systems for bluetongue virus: recovery of infectious virus from synthetic RNA transcripts. J. Virol. 82, 8339–8348 (2008).
Taraporewala, Z. F. et al. Structure-function analysis of rotavirus NSP2 octamer by using a novel complementation system. J. Virol. 80, 7984–7994 (2006).
Knaus, T. & Nassal, M. The encapsidation signal on the hepatitis B virus RNA pregenome forms a stem-loop structure that is critical for its function. Nucleic Acids Res. 21, 3967–3975 (1993).
Porterfield, J. Z. et al. Full-length hepatitis B virus core protein packages viral and heterologous RNA with similarly high levels of cooperativity. J. Virol. 84, 7174–7184 (2010).
Perlman, D. H., Berg, E. A., O'Connor P, B., Costello, C. E. & Hu, J. Reverse transcription-associated dephosphorylation of hepadnavirus nucleocapsids. Proc. Natl Acad. Sci. USA 102, 9020–9025 (2005).
Ahlquist, P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nature Rev. Microbiol. 4, 371–382 (2006).
Wolf, M., Garcea, R. L., Grigorieff, N. & Harrison, S. C. Subunit interactions in bovine papillomavirus. Proc. Natl Acad. Sci. USA 107, 6298–6303 (2010).
Dryden, K. A. et al. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 122, 1023–1041 (1993).
Chappell, J. D., Prota, A. E., Dermody, T. S. & Stehle, T. Crystal structure of reovirus attachment protein σ1 reveals evolutionary relationship to adenovirus fiber. EMBO J. 21, 1–11 (2002).
Acknowledgements
The authors thank N. Leach for careful reading of the manuscript, and S. Harrison, E. Settembre and K. Ogden for helpful discussions. S.D.T. and J.T.P. were supported by the Intramural Research Program of the US National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (grant Z01 AI000788). S.M.M. was supported by the NIAID Intramural Research Program and the Virginia Tech Carilion Research Institute (Roanoke, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Segmented
-
Pertaining to a virus: with a genome comprising multiple distinct nucleic acid molecules; each segment is analogous to a eukaryotic chromosome, but usually encodes only 1–3 proteins.
- Icosahedral
-
With 30 two-fold, 20 three-fold and 12 five-fold axes of rotation (like an icosahedron or a dodecahedron). A type of symmetry common to viral capsids.
- Positive-sense RNAs
-
((+)RNAs). The RNA strands that directly encode the viral proteins and are used as templates to synthesize the negative-sense strands during replication of the genome.
- Triangulation number
-
(T number). The number of symmetrically distinct subunits that make up each of the asymmetric units of the capsid. Generally, larger virions require higher T numbers (that is, more subunits) to form their capsids.
- Five-fold vertices
-
The points on an icosahedron; for viruses of the family Reoviridae, these are the 12 axes of five-fold symmetry and the sites of positive-sense RNA extrusion.
- RNA-capping enzyme
-
An enzyme that is responsible for modifying the 5′ end of an RNA to generate a cap structure that is similar to that of eukaryotic mRNAs.
- Peripentonal channels
-
For rotaviruses, the approximately six-fold-symmetrical 'gaps' in the VP6 layer that surround each of the five-fold vertices. These channels are the binding sites for VP4 trimers during assembly of the viral particle.
- Serotype
-
A classification of rotaviruses using comparative neutralization by monoclonal antibodies; for rotaviruses, there are two outer capsid proteins (VP4 and VP7), giving a binary serotype.
- Neutralizing antibodies
-
Antibodies that block infectivity (for example, of a virus), usually by binding to the foreign particle (the virion) and incapacitating it in some way.
- Galectin
-
A family of sugar-binding proteins that have a similar, distinct fold.
- Sialic acid moieties
-
Carbohydrate functional groups added to proteins or lipids. These groups are used by several viruses (including rotaviruses and influenza viruses) as attachment factors to facilitate attachment to host cells.
- Myristoylated
-
Of a protein: with an amino terminus that has a covalently linked myristic acid fatty acid. This can impart a hydrophobic character to a protein or target it to a membrane.
- Cathepsin
-
A diverse family of intracellular proteases, many members of which function in the low pH environment of the lysosome.
- Negative-sense RNAs
-
The reverse complement of the positive-sense RNAs; for the Reoviridae family viruses, the negative-sense strand is used as a template during transcription to make more positive-sense RNA.
- Reverse genetics systems
-
Methods of specifically modifying a viral genome using recombinant technology (versus a forward genetics screen).
Rights and permissions
About this article
Cite this article
Trask, S., McDonald, S. & Patton, J. Structural insights into the coupling of virion assembly and rotavirus replication. Nat Rev Microbiol 10, 165–177 (2012). https://doi.org/10.1038/nrmicro2673
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2673
This article is cited by
-
Vaccine evaluation and genotype characterization in children infected with rotavirus in Qatar
Pediatric Research (2023)
-
Rotavirus and Norovirus Infections in Children Under 5 Years Old with Acute Gastroenteritis in Southwestern China, 2018–2020
Journal of Epidemiology and Global Health (2022)
-
Genetic characterization of G12P[6] and G12P[8] rotavirus strains collected in six African countries between 2010 and 2014
BMC Infectious Diseases (2021)
-
Recent advances in rotavirus reverse genetics and its utilization in basic research and vaccine development
Archives of Virology (2021)