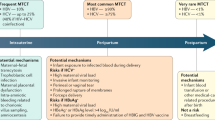
Key Points
-
Perinatal transmission of hepatitis B still occurs worldwide despite the availability of appropriate vaccination
-
High maternal HBV viraemia (>107 copies per ml) is recognized as a risk factor for immunoprophylaxis failure
-
Stratifying the two separate issues of maternal liver disease versus HBV mother-to-child transmission (MTCT) is crucial for clinical decision-making regarding treatment
-
Treatment of confirmed high maternal HBV viraemia in the third trimester might be warranted to reduce the risk of MTCT
Abstract
Chronic HBV infection is estimated to affect >350 million people worldwide and represents a substantial source of morbidity and mortality related to cirrhosis and hepatocellular carcinoma. Mother-to-child transmission (MTCT) remains an important source of incident cases of hepatitis B. Immunoprophylaxis of infants born to mothers who are positive for hepatitis B surface antigen is used to prevent MTCT; however, under-utilization of this intervention in certain regions endemic for HBV infection and failure of immunoprophylaxis in 5–10% of cases are barriers to preventing HBV transmission via this route. Data suggest that a high level of HBV viraemia in pregnant women is a substantial risk factor for immunoprophylaxis failure. Potential means of reducing viral load include antiviral therapy in the third trimester to reduce exposure of the neonate to the virus. Determining the optimal time to treat active HBV-related liver disease in women who wish to become pregnant, as well as managing antiviral therapy in patients who become pregnant, remains challenging. Owing to the vulnerable population affected by these issues, clinical trials are difficult and, thus, evidence-based recommendations are limited. Emerging data are addressing management of HBV during pregnancy that health-care providers should be made aware of. Here, we provide an overview of issues pertinent to HBV infection during pregnancy and present a management algorithm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bosch, F. X. et al. Epidemiology of hepatocellular carcinoma. Clinic Liver Dis. 9, 191–211 (2005).
WHO Fact Sheets. Hepatitis B [online], (2000).
Kowdley, K. V. et al. Prevalence of chronic hepatitis B among foreign born persons living in the United States by country of origin. Hepatology 56, 422–433 (2012).
Devaki, P. et al. Changes in hepatitis B (HBV) vaccination rates and differences in various general demographics across National Health and Nutrition Examination Surveys (NHANES) from 1999–2010. Hepatology 58 (Suppl.), 607A–608A (2013).
Stevens, C. E. et al. HBeAg and anti-HBe detection by radioimmunoassay: correlation with vertical transmission of hepatitis B virus in Taiwan. J. Med. Virol. 3, 237–241 (1979).
Edmunds, W. J. et al. The influence of age on the development of the hepatitis B carrier state. Proc. R. Soc. B. 253, 197–201 (1993).
US Department of Health and Human Services. MMWR: a comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States [online], (2005).
Pan, C. Q. et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B. Clin. Gastroenterol. Hepatol. 10, 452–459 (2012).
Stevens, C. E. et al. Vertical transmission of hepatitis B antigen in Taiwan. N. Engl. J. Med. 292, 771–774 (1975).
Ghaziasadi, A. et al. Mutational analysis of HBsAg-positive mothers and their infected children despite immunoprophylaxis. Iran J. Allergy Asthma Immunol. 12, 352–360 (2013).
Lee, S. D. et al. Maternal hepatitis B virus DNA in mother-infant transmission. Lancet 1, 719 (1989).
Ngui, S. L. et al. Low detection rate and maternal provenance of hepatitis B virus S gene mutants in cases of failed postnatal immunoprophylaxis in England and Wales. J. Infect. Dis. 176, 1360–1365 (1997).
Karthigesu, V. D. et al. A hepatitis B virus variant found in the sera of immunised children induces a conformational change in the HBsAg “a” determinant. J. Med. Virol. 58, 346–352 (1999).
Chiang, C. J. et al. Thirty-year outcomes of the National Hepatitis B Immunization Program in Taiwan. JAMA 310, 974–976 (2013).
US Preventive Services Task Force. Screening for hepatitis B virus infection in pregnancy: US Preventive Services Task Force reaffirmation recommendation statement. Ann. Intern. Med. 150, 869–873 (2009).
Lok, A. S. & McMahon, B. J. AASLD practice guideline update chronic hepatitis B: update 2009. Hepatology 50, 1–36 (2009).
Bai, G. Q., Li, S. H., Yue, Y. F. & Shi, L. The study on role of peripheral blood mononuclear cell in HBV intrauterine infection. Arch. Gynecol. Obstet. 283, 317–321 (2011).
Yu, M. et al. Correlation between vertical transmission of hepatitis B virus and the expression of HBsAg in ovarian follicles and placenta. PLoS ONE 8, e5426 (2013).
Lin, H. H. et al. Transplacental leakage of HBeAg-positive maternal blood as the most likely route in causing intrauterine infection with hepatitis B virus. J. Pediatr. 111, 877–881 (1987).
Lazizi, Y., Badur, S. & Perk, Y. Selective unresponsiveness to HBsAg vaccine in newborns related with an in utero passage of hepatitis B virus DNA. Vaccine 15, 1095–1100 (1997).
Zhang, S. L. et al. Mechanism of Intrauterine infection of hepatitis B virus. World J. Gastroenterology 10, 437–438 (2004).
Xu, D. Z. et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case–control study. J. Med. Virol. 67, 20–26 (2002).
Towers, C. V., Asrat, T. & Rumney, P. The presence of hepatitis B surface antigen and deoxyribonucleic acid in amniotic fluid and cord blood. Am. J. Obstet. Gynecol. 184, 1514–1518 (2001).
Yi, W. et al. Risk of hepatitis B virus vertical transmission after amniocentesis in mothers with chronic hepatitis B. J. Hepatol. http://dx.doi.org/10.1016/j.jhep.2013.11.008.
Wong, V. C., Lee, A. K. & Ip, H. M. Transmission of hepatitis B antigens from symptoms free carrier mothers to the fetus and the infant. Br. J. Obstet. Gynaecol. 87, 958–965 (1980).
Hill, J. B. et al. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet. Gynecol. 99, 1049–1052 (2002).
Mirochnik, M. et al. Antiretrovial concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob. Agents Chemother. 53, 1170–1176 (2009).
Milich, D. & Liang, T. J. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 38, 1075–1086 (2003).
Wang, Z. H. et al. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J. Med. Virol. 71, 360–366 (2003).
Guo, Z. et al. Risk factors of HBV intrauterine transmission among HBsAg-positive pregnant women. J. Viral Hepatol. 20, 317–321 (2013).
Yin, Y. et al. Identification of risk factors associated with immunoprophylaxis failure to prevent the vertical transmission of hepatitis B virus. J. Infect. 66, 447–452 (2013).
Tse, K. et al. Immunoprophylaxis of babies borne to hepatitis B carrier mothers. Hong Kong Med. J. 12, 368–374 (2006).
Burk, R. D. et al. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J. Infect. Dis. 170, 1418–1423 (1994).
Wiseman, E. et al. Perinatal transmission of hepatitis B virus: an Australian experience. MJA 109, 489–492 (2009).
Zou, H. et al. Virologic factors associated with failure to passive–active immunoprophylaxis in infants born to HBsAg-positive mothers. J. Viral Hepatol. 19, e18–e25 (2012).
Yang, J. et al. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus—a systematic review. Virol. J. 5, 100 (2008).
Hu, Y. et al. Effect of elective cesarean section on the risk of mother-to-child transmission of hepatitis B virus. BMC Pregnancy Childbirth 13, 119 (2013).
Pan, C. Q. et al. Cesarean section reduces perinatal transmission of HBV Infection from hepatitis B surface antigen-positive women to their infants. Clin. Gastroenterol. Hepatol. http://dx.doi.org/10.1016/j.cgh.2013.04.026.
Centers for Disease Control and Prevention (CDC) Implementation of newborn hepatitis B vaccination—worldwide, 2006. MMWR Morb. Mortal. Wkly Rep. 46, 1249–1252 (2006).
Tran, T. T. et al. Hepatitis B Research Network (HBRN): maternal knowledge of children's hepatitis B infection and vaccination status [abstract]. Hepatology 58 (Suppl.), 622A–623A (2013).
Singhal, A. et al. Chronic HBV with pregnancy: reactivation flare causing fulminant hepatic failure. Annals Hepatol. 10, 233–236 (2011).
Shaheen, A. A. & Myers, R. P. The outcomes of pregnancy in patients with cirrhosis: a population-based study. Liver Int. 30, 275–283 (2010).
Kim, H. Y. et al. Outcome after discontinuing antiviral agents during pregnancy in women infected with hepatitis B. J. Clin. Virol. 56, 299–305 (2013).
Nguyen, G. et al. Clinical course of hepatitis B virus infection during pregnancy. Aliment. Pharmacol. Ther. 29, 755–764 (2009).
Han, L. et al. A meta-analysis of lamivudine for interruption of mother-to-child transmission of hepatitis B virus. World J. Gastroenterol. 17, 4321–4333 (2011).
Xu, W. M. et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J. Viral Hepat. 16, 94–103 (2009).
Yi, W., Liu, M., Chen, A. & Pan, C. The efficacy of lamivudine use in the second vs. third trimester of pregnancy in preventing vertical transmission of HBV in highly viremic mothers [abstract]. Hepatology 58 (Suppl.) 614A (2013).
Han, G.-R. et al. A prospective and open label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J. Hepatol. 55, 1215–1221 (2011).
Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International. Interim Report for 1 January through 31 January 2013. Wilmington, NC: Registry Coordinating Center; 2013 [online], (2012).
Pan, C. Q. et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin. Gastroenterol. Hepatol. 10, 520–526 (2012).
Liu, M., Cai, H. & Yi, W. Safety of telbivudine treatment for chronic hepatitis B for the entire pregnancy. J. Viral Hepatol. 20 (Suppl. 1), 65–70 (2013).
Han, G. R. et al. Long-term safety and efficacy of infants born to telbivudine-treated highly viremic mothers with HBeAg positive chronic hepatitis B (CHB) during 2nd or 3rd trimester [abstract]. Hepatology 58 (Suppl.), 654A (2013).
Pan, C. Q. et al. Tenofovir disoproxil fumarate for prevention of vertical transmission of hepatitis B virus infection by highly viremic pregnant women: a case series. Dig. Dis. Sci. 57, 2423–2429 (2012).
Pan, C., Liu, M., Cai, H. & Yo, W. Safety of tenofovir disoproxil fumarate (TDF) treatment for the entire pregnancy in mothers with active chronic hepatitis B or cirrhosis [abstract]. Hepatology 58 (Suppl.), 624A (2013).
Greenup, A. J. et al. Efficacy and safety of tenofovir in pregnancy to prevent mother to baby transmission of HBV [abstract]. Hepatology 58 (Suppl.), 625A (2013).
EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection. European Association for the Study of the Liver. J. Hepatol. 57, 167–185 (2012).
Connell, L. E. et al. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 31, 1163–1170 (2011).
Tse, K. Y. et al. The impact of maternal HBsAg carrier status on pregnancy outcomes: A case–control study. J. Hepatol. 43, 771–775 (2005).
Lao, T. T. et al. Maternal hepatitis B surface antigen and incidence of pre-eclampsia. J. Viral Hepat. 20, 343–349 (2013).
Bzowej, N. H. et al. ALT flares are infrequent during the course of pregnancy: perspectives from the Hepatitis B Research Network (HBRN) adult cohort study [abstract]. Hepatology 58 (Suppl.), 646A–647A (2013).
Giles, M. et al. Clinical and virological factors that predict post partum flares in pregnant women with chronic, HBV [abstract]. Hepatology 58 (Suppl.), 638A (2013).
Nguyen, V. et al. Prolonging antiviral therapy after pregnancy for prevention of perinatal HBV transmission does not abrogate post-partum flares [abstract]. Hepatology 58 (Suppl.), 641A (2013).
US Department of Health & Human Services. FDA Viral hepatitis therapies [online], (2013).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects in the production of this article.
Corresponding author
Ethics declarations
Competing interests
T.T.T. acts as a consultant, advisor and/or speaker for Bristol–Myers–Squibb, Gilead Sciences and Novartis. H.P. declares no competing interests.
Rights and permissions
About this article
Cite this article
Patton, H., Tran, T. Management of hepatitis B during pregnancy. Nat Rev Gastroenterol Hepatol 11, 402–409 (2014). https://doi.org/10.1038/nrgastro.2014.30
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.30
This article is cited by
-
Deep sequencing of hepatitis B surface antigen gene in the preserved umbilical cords in immunoprophylaxis failure against mother-to-child HBV transmission
BMC Infectious Diseases (2019)
-
Hepatitis B virus infection
Nature Reviews Disease Primers (2018)
-
Hepatitis B During Pregnancy in Endemic Areas: Screening, Treatment, and Prevention of Mother-to-Child Transmission
Pediatric Drugs (2017)
-
Serum Alanine Aminotransferase and Hepatitis B DNA Flares in Pregnant and Postpartum Women with Chronic Hepatitis B
American Journal of Gastroenterology (2016)
-
Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update
Hepatology International (2016)