Abstract
Few studies were conducted to assess safety and efficacy of continuous antiviral therapy administrated from preconception. In the present study, 136 eligible women with chronic HBV infection were recruited, and assigned to active chronic hepatitis B (CHB) (Group A, B or C) or chronic HBV carrier (Group D). Antiviral therapy was administrated in preconception (Group A), in early (Group B) or late pregnancy (Group C and Group D). Immunoprophylaxis was administrated to all infants. Mothers’ HBV status and ALT were assessed at delivery and 7 months postpartum. Offspring’s HBV status was examined at 7 months old. Group A women showed low HBV DNA level and normal ALT throughout pregnancy. All women at delivery had an HBV DNA level of less than 106 IU/ml, but the proportion of patients with lower HBV DNA level in Group A was higher than any of other three groups (P < 0.05). No differences in obstetrical complications were found among the four groups. None of infants who completed follow-up showed positive HBsAg at age of 7 months. Congenital malformation and infant growth indicators were similar among study cohorts. Continuous antiviral therapy from preconception to entire pregnancy is effective and safe for active CHB mothers and their infants.
Similar content being viewed by others
Introduction
Chronic HBV infection, a major cause for cirrhosis and hepatocellular carcinoma (HCC), remains an important public health issue worldwide, particularly in Asia and Africa1. Approximately 3.6% of the global population are chronically infected with HBV through perinatal transmission, a leading HBV transmission route2. Approximately 2 million children are newly infected with HBV every year through mother-to-child transmission (MTCT)3. Unfortunately, 90% of these children become chronic HBV infection that may possibly develop into progressive liver fibrosis, cirrhosis, even HCC in years or decades4. Therefore, blocking MTCT is a vital measure in reducing overall HBV prevalence and health burden.
The combined immunization of hepatitis B vaccine and hepatitis B immunoglobulin (HBIG) is generally used to prevent MTCT of HBV4. However, immunoprophylaxis fails in about 2–10% of infants5,6, whose mothers are with hepatitis B e antigen (HBeAg) positive or high viremia7,8. The failure rate can even increase to 30% for infants born to mothers with high HBV DNA level (HBV DNA ≥ 106 IU/ml)9,10,11. Therefore, controlling maternal HBV DNA level is a critical measure for preventing MTCT of HBV. For mothers with high HBV DNA level, use of telbivudine (LDT)12 or tenofovir disoproxil fumarate (TDF)13 during early or late pregnancy to suppress viral replication, in combination with immunoprophylaxis, is considered to be an important approach to reducing MTCT of HBV12,13,14. However, some infants born to mothers undergoing antiviral therapy during the second or third trimester of pregnancy were infected with HBV15, suggesting that antiviral prophylaxis should start earlier. However, the best or optimal time for starting antiviral treatment remains uncertain.
Some child-bearing women with active chronic hepatitis B (CHB) should take oral antiviral drugs in preconception, because antiviral treatment for chronic HBV infection could control hepatitis flare and reduce the incidence of cirrhosis, HCC, and death16,17. These patients have to take oral antiviral drugs for a long time period, even lifelong, because oral antiviral drugs only inhibit HBV rather than kill HBV. However, hepatitis flare and virological recurrence are common after discontinuity of antiviral treatment18,19. Therefore, a long-term treatment is indispensable to maintain a virological response. Furthermore, HBV can be vertically transmitted to the offspring via germ cells20. It remains unclear whether the patients should continuously take antiviral drugs throughout pregnancy when their liver enzyme turns to be normal prior to becoming pregnant. To date, antiviral therapy is usually initiated after a child-bearing woman becomes pregnant. Few clinical studies about antiviral therapy were carried out during the embryo implantation phase. In the present study, we assessed whether continuous antiviral therapy initiated from preconception through the entire pregnancy is safe and effective to reduce MTCT of HBV.
Results
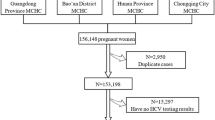
From January 1st, 2017 to December 31st 2018, a total of 164 pregnant women were screened. A total of 136 eligible pregnant women were then enrolled and assigned to active chronic hepatitis B (CHB) groups (i.e., Groups A, B, or C) or chronic HBV carrier group (Group D). The antiviral therapy was administrated in preconception (Group A, 25 cases), in early (Group B, 17 cases) or late pregnancy (Group C, 34 cases and Group D, 60 cases), respectively (Fig. 1). Finally, we obtained the successful follow-up rates ranging from 85.7–96.9% for those four study groups.
Efficacy and safety of antiviral therapy for mother
As shown in Table 1, there was no statistically significant difference in maternal age among these groups. In each group, the majority of enrolled cases were positive for hepatitis B e antigen (HBeAg). Group A patients showed a low HBV DNA level and normal ALT throughout pregnancy due to their initiation of antiviral treatment during preconception and continuity of the regimen throughout pregnancy. At delivery, the proportion of patients with low HBV DNA level in Group A was higher than any of other three groups (P < 0.05). Furthermore, all pregnant women in the four study groups had an HBV DNA level of less than 106 IU/ml at delivery. Three of 17(17.6%) patients in Group B, 10 of 34 (29.4%) in Group C and 4 of 60 (6.7%) in Group D presented with abnormal ALT at delivery, but all Group A patients showed normal ALT at delivery.
There were no statistically significant differences in delivery mode and gestational age among the four groups (Table 2). There were also no statistically significant differences in selected complications of pregnancy among the four study groups (P > 0.05).
Safety of antiviral therapy for fetus and infant
As shown in Table 3, there were no statistically significant differences in fetal development indicators and infants’ growth for the four groups (P > 0.05). Birth weight, body length and Apgar scores at 1 min were similar among the four groups (P > 0.05). There were no statistically significant differences in neonatal complications including congenital malformations, preterm birth and fetal/neonatal mortality (P > 0.05). There were no statistically significant differences in infants’ weight, length, abnormal development at age of 6 months among the four groups (P > 0.05).
Effectiveness of antiviral therapy on MTCT of HBV
The MTCT rates of HBV for the four follow-up groups were shown in Table 4. None of infants who underwent the follow-up was positive for hepatitis B surface antigen (HBsAg) while all infants showed positive hepatitis B surface antibody (HBsAb). Group B showed the highest positive rate of antibody to hepatitis virus B core antigen (HBcAb), Group D showed the lowest (P < 0.05). Group A showed the lowest positive rate of HBcAb among the three active CHB groups.
Discussion
In the present study, we found that Group A patients showed low HBV DNA level and normal ALT throughout pregnancy. The proportion of patients with lower HBV DNA level in Group A was higher than any of other three groups (P < 0.05). At delivery, all Group A patients were with normal ALT, but some patients of other three groups were with abnormal ALT. Compared with patients who took antiviral drugs from late pregnancy (Groups C and D), patients who took antiviral drugs from preconception or early pregnancy (Groups A and B) did not show difference in obstetrical complications. Furthermore, their congenital malformation and neonatal growth indicators were similar among four groups. It is already confirmed that antiviral therapy initiated in the second and third trimesters of pregnancy is effective to prevent perinatal transmission of HBV12,13,14. Unfortunately, some infants born to mothers undergoing antiviral therapy during the second or third trimester of pregnancy were infected with HBV13,15. As a result, the most effective time to start an antiviral treatment is unknown. Our results showed that for pregnant women with active chronic hepatitis B (CHB), continuous antiviral treatment from preconception through the entire pregnancy is safe for both mothers and infants, and an effective means to reduce HBV entering offspring’s body.
In our study, we focused on the active CHB patients and chronic HBV carriers with high HBV DNA level, as they all should be given antiviral therapy to control hepatitis flare and reduce MTCT of HBV. Compared with Group C or Group D (antiviral therapy administrated in late pregnancy), other two groups (Group A, antiviral therapy from preconception; Group B, antiviral therapy in early pregnancy) did not present with significant maternal and infants’ complications (P > 0.05). Our results indicate that taking TDF/LDT therapy could be safe for the mothers and their offspring from preconception through the entire pregnancy. Although our study sample sizes were relatively small, those pregnant women had comparable characteristics (e.g., small age difference and unique Chinese ethnicity), and we used consistent laboratory test methods throughout the study. In the future study, more pregnant women may be needed to determine whether there may be possible effect of antiviral drugs on the congenital abnormality or birth defect. Furthermore, because the following-ups only were carried out within 28–36 weeks postpartum in previous studies13,21,22, our evaluation on the safety might be incomplete, thus the follow-up period needs to be extended.
Continuous antiviral treatment from preconception though the entire pregnancy is very important for active CHB patients to keep liver function normal and control maternal liver damage23. The cessation of antiviral therapy could result in severe exacerbations, even liver failure for CHB patients24. Compared with non-pregnant women, pregnant women are more susceptible to liver failure due to chronic HBV infection, because HBV infection is the most important causes of severe hepatitis during pregnancy in China25. ALT levels and HBV DNA load levels could be enhanced during the entire pregnancy, because the alterations immune regulation could contribute to the increase of HBV replication and ALT level during pregnancy26,27,28. Furthermore, HBV and ALT flares could even be severe during pregnancy29. CHB patients are liable to develop into liver fibrosis, even cirrhosis if their ALT flares are repeated and severe30. As a result, some pregnant women with CHB could develop into severe liver fibrosis, cirrhosis during pregnancy. In our study Group A (antiviral therapy from preconception), patients presented with normal ALT throughout pregnancy might be due to initiation of antiviral treatment during preconception and continuation of the regimen through the entire pregnancy. In our Group B and Group C, these patients’ liver function gradually turned to be normal after undergoing antiviral therapy. None of the patients developed into liver failure or cirrhosis. However, one patient in Group B and one in Group C were both excluded due to their own drug withdrawal. The two patients showed normal ALT while they took antiviral drugs. The ALT was 544 U/liter and 422.2 U/liter, respectively, after their withdrawing antiviral drug. However, ALT level gradually returned to normal after they took antiviral drug again.
Continuous antiviral treatment starting in preconception appears to be an effective means to control maternal hepatic damage and reduce HBV transmission from mother to the fetus. Compared with maternal HBV DNA level and ALT level of other groups at delivery (Table 1), Group A patients (antiviral therapy from preconception) were with lower HBV DNA level and normal ALT throughout the pregnancy, showing that continuous antiviral treatment from preconception through the entire pregnancy effectively have reduced maternal HBV DNA level and hepatic damage. The lower maternal HBV DNA level was, the lower the risk of HBV entering offspring’s body was. The lower the risk of HBV entering offspring’s body was, the higher the success rate of infant immunoprophylaxis was. Additionally, in the present study, none of infants that completed follow-up showed HBsAg positive, and all infants showed HBsAb positive. None of infants in our study was infected with HBV. It is generally accepted that maternal HBV DNA level plays a vital role in MTCT of HBV4,9,10,11. Several recent studies13,14,21 reported that MTCT rate of HBV was reduced if patients took antiviral therapy (e.g., LAM, LDT and TDF) during the late pregnancy. Our results also demonstrated that antiviral therapy during pregnancy decreased MTCT rate of HBV. Moreover, in our study, some infants with HBcAb positive, HBsAb positive and HBsAg negative indicated that even if MTCT of HBV did not occur, HBV still entered some offsprings’ body. Because the HBcAb positive rate at age of 0–24 months might partly result from the passive transfer of their mothers’ body or mean that HBV has ever entered into infants’ body31,32, we would further study the sense of HBcAb to infants.
In previous studies13,22, antiviral therapy continued to 1 month postpartum to interrupt MTCT of HBV. In our study, patients with high HBV DNA level and normal liver function during pregnancy immediately discontinued antiviral therapy after delivery. Then these mothers could breast-feed their infants. Although EASL guideline in 201719 recommended that patients who continued TDF therapy postpartum should be advised to breast-feed, most mothers who continued TDF therapy after delivery remained unwilling to breast-feed because they were afraid of any potential adverse effect of TDF on their infants in our clinical practice. Furthermore, TDF drug labels, the clinical guidelines of the Chinese Society of Hepatology33, and the clinical guidelines of Asian Pacific34 do not clearly recommend that breastfeeding be encouraged while the mother undergoes TDF treatment.
In summary, our findings suggest that continuous antiviral treatment from preconception through the entire pregnancy is safe for active CHB mothers and their infants. This effective means can reduce HBV entering offspring’s body. Continuous antiviral therapy throughout pregnancy can prevent MTCT of HBV. Furthermore, antiviral therapy appears to control hepatitis flare and promote ALT normalization during pregnancy. Initiation of antiviral therapy for prospective pregnant women with active CHB may be advanced to preconception.
Methods
Patients
The current prospective cohort study was approved by the institutional ethics review committee of the Third Affiliated Hospital of Guangzhou Medical University, and was carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed written consent was obtained from each patient in the present study. The present study was registered in ClinicalTrials.gov (No. NCT03181607). We had access to the study data and had reviewed and approved the final manuscript.
This multicenter cohort study was conducted between January 2017 and December 2018. Pregnant women with chronic HBV infection, who visited six hospitals in China and met the following inclusion criteria and exclusion criteria, were enrolled on a voluntary basis. The inclusion criteria were as follows: (a) a history of HBV infection ≥ 6 months; (b) positive for HBsAg; (c) for patients already administrated with antiviral treatment in preconceptional period, the therapy could not be discontinued; for patients never administrated with antiviral treatment, ALT ≥ 2 times the upper limit of normal (ULN), or HBV DNA level ≥ 104 IU/ml (positive for HBeAg) or HBV DNA level ≥ 103 IU/ml (negative for HBeAg), traditional protecting liver and reducing enzyme treatment failed; (d) good compliance of patients.
The exclusion criteria were as follows: (a) patients with antibodies against HIV, HCV, HDV, or other forms of chronic liver disease; (b) evidence of hepatocellular carcinoma, decompensated liver disease, auto-immune hepatitis, or significant renal, cardiovascular, respiratory or neurological comorbidity; (c) concurrent treatment with nephrotoxic drugs, glucocorticoids, cytotoxic drugs, non-steroidal anti-inflammatory drugs, or immune modulators; (d) ultra-sonographic evidence of fetal malformation, abnormal fetal development or placental abnormality; (e) clinical signs of threatened miscarriage; (f) a history of complicated pregnancy; (g) drug discontinuance by themselves.
Study design
As shown in Fig. 1, according to the inclusion criteria and exclusion criteria, 136 eligible pregnancy women with chronic HBV infection were enrolled in our study. They were assigned to the following four groups. In group A, patients with active CHB received an oral dose of 300 mg of tenofovir disoproxil fumarate (TDF, GlaxoSmithKline) or 600 mg of telbivudine (LDT, Novartis China) once per day due to ever abnormal liver function. When their liver enzyme turned to be normal prior to pregnancy, they took TDF or LDT continuously during their entire pregnancy. In group B and group C, pregnancies with CHB showed abnormal liver function in early pregnancy (less than 24 gestational weeks) and in late pregnancy (more than 24 gestational weeks), respectively. Then they began to take TDF (300 mg/times) or LDT (600 mg/times) every day, respectively. In group D, the pregnant women showed high HBV DNA level (≥ 106 IU/ml) and normal liver function. To reduce HBV DNA level and risk of MTCT, they were treated with TDF (orally 300 mg/times, Qd) or LDT (orally 600 mg/times, Qd) during the late pregnancy.
During pregnancy, all patients were followed up every 4 weeks to monitor adverse events and laboratory results (liver function tests, HBV DNA level, hematologic tests, etc.). Following delivery, patients in Groups A, B and C continued to receive TDF/LDT therapy, and patients in Group D stopped receiving TDF/LDT therapy. All these patients were also followed up every 4 weeks in the first 3 months of delivery. They subsequently were followed up at 24th week and 28th week postpartum, respectively.
All infants received 100 IU of hepatitis B immune globulin (HBIG, Hualan Biotechnology Co., Ltd. Xinxiang City, Henan Province, China) and 10 μg of the recombined hepatitis B vaccine (RHBV, Shenzhen Kangtai Biotechnology Co., Ltd., Shenzhen City, Guangdong Province, China) intramuscularly within 1–24 h after birth. The same dose of HBIG was administrated at age of 4 weeks. The same dose of recombined hepatitis B vaccine was given at week 4 and week 24.
All mothers and their infants were followed up to 7 months postpartum.
Outcome measures
The primary outcome included the efficacy and the safety of antiviral therapy for mothers. We analyzed maternal HBV DNA level and alanine transaminase (ALT) at delivery and 7 months postpartum. To measure the safety of antiviral therapy on the mothers, adverse complications of mothers were analyzed.
The secondary outcome included the effectiveness of blocking MTCT of HBV and the safety of TDF/LDT for the offspring. Fetal development indicators such as birth weight, body length and congenital abnormalities at birth and at age of 6 months were evaluated to assess the safety of antiviral therapy with respect to fetal complications. HBV antigen and antibody status of infants were assayed at age of 7 months to assess the effectiveness of blocking MTCT of HBV.
Statistical analyses
All data were analyzed using SPSS v22.00 statistical analysis software (SPSS Inc, Chicago, IL). Descriptive variables were expressed as mean ± standard deviation (SD) or percentage. Analysis of variance (ANOVA) test and χ2 test were used to compare quantitative and categorical variables, respectively. Differences were considered statistically significant at a P value < 0.05.
Conclusion
Our findings from this clinical trial apparently support our hypothesis that continuous antiviral treatment from preconception through the entire pregnancy is safe for active CHB mothers and their infants. Antiviral therapy appears to control hepatitis flare and promote ALT normalization during pregnancy. Initiation of antiviral therapy for prospective pregnant women with active CHB may be advanced to preconception.
References
Indolfi, G. et al. Hepatitis B Virus Infection in Children and Adolescents. Lancet Gastroenterol. Hepatol.4, 466–476 (2019).
Schweitzer, A., Horn, J., Mikolajczyk, R. T., Krause, G. & Ott, J. J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet386, 1546–1555 (2015).
Prevalence, G. Treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol.3, 383–403 (2018).
Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection, World Health Organization (Geneva, 2015).
Guo, Z. et al. Risk factors of HBV intrauterine transmission among HBsAg-positive pregnant women. J. Viral. Hepat.20, 317–321 (2013).
Zou, H., Chen, Y., Duan, Z., Zhang, H. & Pan, C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J. Viral. Hepat.19, e18–e25 (2012).
Lin, X. et al. Immunoprophylaxis failure against vertical transmission of hepatitis B virus in the Chinese population: a hospital-based study and a meta-analysis. Pediatr. Infect. Dis. J.33, 897–903 (2014).
Liu, C. P. et al. Factors associated with mother-to-child transmission of hepatitis B virus despite immunoprophylaxis. Int. Med.54, 711–716 (2015).
Sellier, P. et al. Untreated highly viraemic pregnant women from asia or sub-Saharan Africa often transmit hepatitis B virus despite serovaccination to newborns. Liver Int.35, 409–416 (2015).
Vodkin, I. & Patton, H. Management of hepatitis B virus infection during pregnancy. Minerva Gastroenterol. Dietol.60, 205–214 (2014).
Pan, C. Q. et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol. Hepatol.10, 452–459 (2012).
He, T. et al. Safety and efficacy of lamivudine or telbivudine started in early pregnancy for mothers with active chronic hepatitis B. Hepatol. Int.12, 118–125 (2018).
Pan, C. Q. et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N. Engl. J. Med.374, 2324–2334 (2016).
Hu, Y. et al. Safety and efficacy of telbivudine in late pregnancy to prevent mother-to-child transmission of hepatitis B virus: a multicenter prospective cohort study. J. Viral. Hepat.25, 429–437 (2018).
Liu, J. et al. Efficacy and safety of telbivudine and tenofovir disoproxil fumarate in preventing hepatitis B vertical transmission: a real-life practice. J. Viral. Hepat.26(10), 1170–1177 (2019).
Liaw, Y. F. et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med.351, 1521–1531 (2004).
Thomas, D. L. Global elimination of chronic hepatitis. N. Engl. J. Med.380, 2041–2050 (2019).
Terrault, N. A. et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology63, 261–283 (2016).
EASL. Clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol.67, 370–398 (2017).
Hu, X. L. et al. The presence and expression of the hepatitis B virus in human oocytes and embryos. Hum. Reprod.26, 1860–1867 (2011).
Khalighinejad, P., Alavian, S. M., Fesharaki, M. G. & Jalilianhasanpour, R. Lamivudine’s efficacy and safety in preventing mother-to-child transmission of hepatitis B: a meta-analysis. Turk. J. Gastroenterol.30, 66–74 (2019).
Yi, W. et al. Prospective cohort study on the efficacy and safety of telbivudine used throughout pregnancy in blocking mother-to-child transmission of hepatitis B virus. J Viral Hepat.24(Suppl 1), 49–56 (2017).
Kim, H. Y. et al. Outcome after discontinuing antiviral agents during pregnancy in women infected with hepatitis B virus. J. Clin. Virol.56, 299–305 (2013).
Su, G. G. et al. Efficacy and safety of lamivudine treatment for chronic hepatitis B in pregnancy. World J. Gastroenterol.10, 910–912 (2004).
Yang, Y. B., Li, X. M., Shi, Z. J. & Ma, L. Pregnant woman with fulminant hepatic failure caused by hepatitis B virus infection: a case report. World J. Gastroenterol.10, 2305–2306 (2004).
Chang, C. Y. et al. Serum alanine aminotransferase and hepatitis B DNA flares in pregnant and postpartum women with chronic hepatitis B. Am. J. Gastroenterol.111, 1410–1415 (2016).
Tan, H. H., Lui, H. F. & Chow, W. C. Chronic hepatitis B virus (HBV) infection in pregnancy. Hepatol. Int.2, 370–375 (2008).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol.5, 266–271 (2004).
Aslam, A., Campoverde, R. K., Malladi, V. R., Ishtiaq, R. & Lau, D. Management of chronic hepatitis B during pregnancy. Gastroenterol. Rep. (Oxf).6, 257–262 (2018).
Chang, M. L. & Liaw, Y. F. Hepatitis B flares in chronic Hepatitis B: pathogenesis, natural course, and management. J. Hepatol.61, 1407–1417 (2014).
Chen, H. L. et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology142, 773–781 (2012).
Lee, P. I., Lee, C. Y., Huang, L. M. & Chang, M. H. Long-term efficacy of recombinant hepatitis B vaccine and risk of natural infection in infants born to mothers with hepatitis B E antigen. J. Pediatr.126, 716–721 (1995).
Hou, J. et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update). J. Clin. Transl. Hepatol.5, 297–318 (2017).
Sarin, S. K. et al. Asian-pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int.10, 1–98 (2016).
Acknowledgements
The present study was funded by the National Key Research and Development Program of China (2017YFC1001402, 2018YFC1002900), National Natural Science Foundation of China (81401306, 81830045) and Clinical Research Project of Guangzhou Medical University (No. 2017[160]).
Author information
Authors and Affiliations
Contributions
X.P. and J.C. conceived and designed the study. L.Z., X.O., S.Z., J.C., F.H., Y.L., H.W., B.C., Z.W., H.L., G.L. and Z.H. acquired data. X.P., J.C., F.H., Y.L., H.W., B.C., Z.W., H.L., G.L. and Z.H. analyzed and interpreted data. X.P. and J.C. wrote the manuscript. S.L., G.S. and D.C. critically revised the manuscript for important intellectual content. X.P., J.C. and D.C. used statistical analysis software to calculate data. X.P., J.C. and D.C. obtained funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, X., Chen, J., Zhou, L. et al. Efficacy and safety of continuous antiviral therapy from preconception to prevent perinatal transmission of hepatitis B virus. Sci Rep 10, 13631 (2020). https://doi.org/10.1038/s41598-020-70644-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70644-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.