Key Points
-
Humans are continuously exposed to numerous chemicals, including endocrine-disrupting chemicals (EDCs), throughout life
-
The ovarian dysgenesis syndrome hypothesis proposes that exposure to certain chemicals, including EDCs, during fetal life can lead to reproductive disorders later in life
-
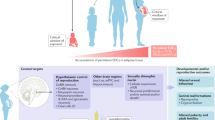
Female fetuses are particularly sensitive to chemical exposure during four developmental windows: early gonadal development, meiotic division of germ cells, follicular assembly and early follicle recruitment
-
Some human epidemiological data suggest that the incidence of female reproductive disorders has increased since the mid-1950s and that EDCs are potential contributors
Abstract
A woman's reproductive health and ability to have children directly affect numerous aspects of her life, from personal well-being and socioeconomic standing, to morbidity and lifespan. In turn, reproductive health depends on the development of correctly functioning ovaries, a process that starts early during fetal life. Early disruption to ovarian programming can have long-lasting consequences, potentially manifesting as disease much later in adulthood. A growing body of evidence suggests that exposure to chemicals early in life, including endocrine-disrupting chemicals, can cause a range of disorders later in life, such as those described in the ovarian dysgenesis syndrome hypothesis. In this Review, we discuss four specific time windows during which the ovary is particularly sensitive to disruption by exogenous insults: gonadal sex determination, meiotic division, follicle assembly and the first wave of follicle recruitment. To date, most evidence points towards the germ cell lineage being the most vulnerable to chemical exposure, particularly meiotic division and follicle assembly. Environmental chemicals and pharmaceuticals, such as bisphenols or mild analgesics (including paracetamol), can also affect the somatic cell lineages. This Review summarizes our current knowledge pertaining to environmental chemicals and pharmaceuticals, and their potential contributions to the development of ovarian dysgenesis syndrome. We also highlight knowledge gaps that need addressing to safeguard female reproductive health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hunt, P. A., Sathyanarayana, S., Fowler, P. A. & Trasande, L. Female reproductive disorders, diseases, and costs of exposure to endocrine disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 101, 1562–1570 (2016).
World Health Organization. Global assessment of the state-of-the-science of endocrine disruptors. WHO http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/ (2002).
Skakkebaek, N. E., Rajpert-De Meyts, E. & Main, K. M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 (2001).
Buck Louis, G. M., Cooney, M. A. & Peterson, C. M. The ovarian dysgenesis syndrome. J. Dev. Orig. Health Dis. 2, 25–35 (2011).
Crain, D. A. et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 90, 911–940 (2008).
Gore, A. C. et al. EDC-2: the Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 36, E1–E150 (2015).
Fowler, P. A. et al. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol. Cell. Endocrinol. 355, 231–239 (2012).
Reed, C. E. & Fenton, S. E. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res. C Embryo Today 99, 134–146 (2013).
Palmer, J. R. et al. Infertility among women exposed prenatally to diethylstilbestrol. Am. J. Epidemiol. 154, 316–321 (2001).
Hatch, E. E. et al. Age at natural menopause in women exposed to diethylstilbestrol in utero. Am. J. Epidemiol. 164, 682–688 (2006).
Steiner, A. Z., D'Aloisio, A. A., DeRoo, L. A., Sandler, D. P. & Baird, D. D. Association of intrauterine and early-life exposures with age at menopause in the Sister Study. Am. J. Epidemiol. 172, 140–148 (2010).
Hoover, R. N. et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N. Engl. J. Med. 365, 1304–1314 (2011).
Mahalingaiah, S. et al. Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the Nurses' Health Study II. Am. J. Epidemiol. 179, 186–191 (2014).
Baird, D. D. & Newbold, R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod. Toxicol. 20, 81–84 (2005).
Missmer, S. A. et al. In utero exposures and the incidence of endometriosis. Fertil. Steril. 82, 1501–1508 (2004).
Laronda, M. M., Unno, K., Butler, L. M. & Kurita, T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation 84, 252–260 (2012).
Jensen, T. K. et al. Early exposure to smoking and future fecundity among Danish twins. Int. J. Androl. 29, 603–613 (2006).
Kristensen, S. L. et al. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum. Reprod. 28, 3337–3348 (2013).
Kristensen, S. L. et al. Prenatal exposure to persistent organochlorine pollutants and female reproductive function in young adulthood. Environ. Int. 92–93, 366–372 (2016).
Palioura, E. & Diamanti-Kandarakis, E. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev. Endocr. Metab. Disord. 16, 365–371 (2015).
Chao, H.-H. et al. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem. Cell Biol. 137, 249–259 (2012).
Johansson, H. K. L. et al. Perinatal exposure to mixtures of endocrine disrupting chemicals reduces female rat follicle reserves and accelerates reproductive aging. Reprod. Toxicol. 61, 186–194 (2016).
Rodríguez, H. A., Santambrosio, N., Santamaría, C. G., Muñoz-de-Toro, M. & Luque, E. H. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod. Toxicol. 30, 550–557 (2010).
Zhang, X.-F. et al. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ. Mol. Mutagen. 54, 354–361 (2013).
Faubion, S. S., Kuhle, C. L., Shuster, L. T. & Rocca, W. A. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 18, 483–491 (2015).
Fernández, M., Bourguignon, N., Lux-Lantos, V. & Libertun, C. Neonatal exposure to bisphenol A and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ. Health Perspect. 118, 1217–1222 (2010).
Gao, H. et al. Bisphenol A and hormone-associated cancers: current progress and perspectives. Medicine (Baltimore). 94, e211 (2015).
Susiarjo, M., Hassold, T. J., Freeman, E. & Hunt, P. A. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 3, e5 (2007). This study revealed that fetal exposure to BPA disturbs both synapsis and recombination during meiotic prophase I and that the effect can be mediated by antagonism of ER β.
Wang, W., Hafner, K. S. & Flaws, J. A. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol. Appl. Pharmacol. 276, 157–164 (2014).
Richardson, B. E. & Lehmann, R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat. Rev. Mol. Cell Biol. 11, 37–49 (2010).
Svingen, T. & Koopman, P. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 27, 2409–2426 (2013).
Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117–121 (1991).
Eggers, S. & Sinclair, A. Mammalian sex determination — insights from humans and mice. Chromosome Res. 20, 215–238 (2012).
Nicol, B. & Yao, H. H.-C. Building an ovary: insights into establishment of somatic cell lineages in the mouse. Sex. Dev. 8, 243–251 (2014).
Wilhelm, D., Palmer, S. & Koopman, P. Sex determination and gonadal development in mammals. Physiol. Rev. 87, 1–28 (2007).
Koopman, P. The delicate balance between male and female sex determining pathways: potential for disruption of early steps in sexual development. Int. J. Androl. 33, 252–258 (2010).
Holm, J. B. et al. Intrauterine exposure to paracetamol and aniline impairs female reproductive development by reducing follicle reserves and fertility. Toxicol. Sci. 150, 178–189 (2016). This was the first study to show that exposure to paracetamol during fetal life can have negative consequences for ovary development in mice, resulting in a reduction in the number of primordial and growing follicles and in total number of follicles by 7 weeks, and in a further reduction in fertility by 6 months and 10 months of age.
Bayne, R. A. L. et al. Prostaglandin E2 as a regulator of germ cells during ovarian development. J. Clin. Endocrinol. Metab. 94, 4053–4060 (2009).
Dean, A. et al. Analgesic exposure in pregnant rats affects fetal germ cell development with inter-generational reproductive consequences. Sci. Rep. 6, 19789 (2016). This convincing study showed that fetal exposure to paracetamol or indomethacin can delay meiotic entry, reduce the number of germ cells and ultimately affect fertility in adult female rats.
Kristensen, D. M. et al. Analgesic use — prevalence, biomonitoring and endocrine and reproductive effects. Nat. Rev. Endocrinol. 12, 381–393 (2016).
Yu, M. et al. Effects of tamoxifen on the sex determination gene and the activation of sex reversal in the developing gonad of mice. Toxicology 321, 89–95 (2014).
Wilhelm, D. et al. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech. Dev. 126, 324–336 (2009).
Hersmus, R. et al. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J. Pathol. 215, 31–38 (2008).
Lindeman, R. E. et al. Sexual cell-fate reprogramming in the ovary by DMRT1. Curr. Biol. 25, 764–771 (2015).
Ottolenghi, C. et al. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 16, 2795–2804 (2007).
Uhlenhaut, N. H. et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130–1142 (2009).
Zhao, L., Svingen, T., Ng, E. T. & Koopman, P. Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development 142, 1083–1088 (2015).
Crisponi, L. et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat. Genet. 27, 1 59–166 (2001).
Couse, J. F. et al. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and ß. Science 286, 2328–2331 (1999).
Brandenberger, A. W., Tee, M. K., Lee, J. Y., Chao, V. & Jaffe, R. B. Tissue distribution of estrogen receptors alpha (ER-α) and beta (ER-β) mRNA in the midgestational human fetus. J. Clin. Endocrinol. Metab. 82, 3509–3512 (1997).
Jefferson, W. N., Couse, J. F., Banks, E. P., Korach, K. S. & Newbold, R. R. Expression of estrogen receptor is developmentally regulated in reproductive tissues of male and female mice. Biol. Reprod. 62, 310–317 (2000).
Aoki, T. & Takada, T. Bisphenol A modulates germ cell differentiation and retinoic acid signaling in mouse ES cells. Reprod. Toxicol. 34, 463–470 (2012).
Spiller, C. M. & Bowles, J. Sex determination in mammalian germ cells. Asian J. Androl. 17, 427–432 (2015).
Bowles, J. et al. Retinoid signaling determines germ cell fate in mice. Science 312, 596–600 (2006).
Koubova, J. et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl Acad. Sci. USA 103, 2474–2479 (2006).
McLaren, A. & Southee, D. Entry of mouse embryonic germ cells into meiosis. Dev. Biol. 187, 107–113 (1997).
Di Giacomo, M. et al. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc. Natl Acad. Sci. USA 102, 737–742 (2005).
Xu, H., Beasley, M. D., Warren, W. D., van der Horst, G. T. J. & McKay, M. J. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev. Cell 8, 949–961 (2005).
Li, X. C., Bolcun-Filas, E. & Schimenti, J. C. Genetic evidence that synaptonemal complex axial elements govern recombination pathway choice in mice. Genetics 189, 71–82 (2011).
Hunt, P. A. et al. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 13, 546–553 (2003).
Hunt, P. A. et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl Acad. Sci. USA 109, 17525–17530 (2012).
Fowler, P. A. et al. Development of steroid signaling pathways during primordial follicle formation in the human fetal ovary. J. Clin. Endocrinol. Metab. 96, 1754–1762 (2011).
Zhang, H.-Q. et al. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol. Biol. Rep. 39, 5651–5657 (2012).
Lawson, C. et al. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol. Reprod. 84, 79–86 (2011).
Zhang, X.-F. et al. Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod. Fertil. Dev. 27, 1213–1221 (2015).
Covaci, A. et al. Urinary BPA measurements in children and mothers from six European member states: overall results and determinants of exposure. Environ. Res. 141, 77–85 (2015).
LaKind, J. S. & Naiman, D. Q. Temporal trends in bisphenol A exposure in the United States from 2003–2012 and factors associated with BPA exposure: spot samples and urine dilution complicate data interpretation. Environ. Res. 142, 84–95 (2015).
Frederiksen, H. et al. Bisphenol A and other phenols in urine from Danish children and adolescents analyzed by isotope diluted TurboFlow-LC–MS/MS. Int. J. Hyg. Environ. Health 216, 710–720 (2013).
Pepling, M. E. & Spradling, A. C. Female mouse germ cells form synchronously dividing cysts. Development 125, 3323–3328 (1998).
Pepling, M. E. et al. Differences in oocyte development and estradiol sensitivity among mouse strains. Reproduction 139, 349–357 (2010).
Pepling, M. E. & Spradling, A. C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 (2001).
Gawriluk, T. R. et al. Autophagy is a cell survival program for female germ cells in the murine ovary. Reproduction 141, 759–765 (2011).
Escobar, M. L., Echeverría, O. M., Ortíz, R. & Vázquez-Nin, G. H. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis 13, 1253–1266 (2008).
Grive, K. J. & Freiman, R. N. The developmental origins of the mammalian ovarian reserve. Development 142, 2554–2563 (2015).
Chen, Y., Jefferson, W. N., Newbold, R. R., Padilla-Banks, E. & Pepling, M. E. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148, 3580–3590 (2007).
Jefferson, W., Newbold, R., Padilla-Banks, E. & Pepling, M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol. Reprod. 74, 161–168 (2006).
Kezele, P. & Skinner, M. K. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 144, 3329–3337 (2003).
Pepe, G. J., Billiar, R. B. & Albrecht, E. D. Regulation of baboon fetal ovarian folliculogenesis by estrogen. Mol. Cell. Endocrinol. 247, 41–46 (2006).
Fowler, P. A. et al. Gene expression analysis of human fetal ovarian primordial follicle formation. J. Clin. Endocrinol. Metab. 94, 1427–1435 (2009).
Fowler, P. A. et al. In utero exposure to cigarette smoke dysregulates human fetal ovarian developmental signalling. Hum. Reprod. 29, 1471–1489 (2014).
Karavan, J. R. & Pepling, M. E. Effects of estrogenic compounds on neonatal oocyte development. Reprod. Toxicol. 34, 51–56 (2012). This study showed that, by exposing neonatal mice to diethylstilbestrol, ethinyl oestradiol or BPA, the rate of apoptosis decreases and the number of single oocytes is reduced, whereas the total number of oocytes per section and the number of primordial follicles increase.
Chalmey, C. et al. Systemic compensatory response to neonatal estradiol exposure does not prevent depletion of the oocyte pool in the rat. PLoS ONE 8, e82175 (2013).
Wang, C. & Roy, S. K. Development of primordial follicles in the hamster: role of estradiol-17β. Endocrinology 148, 1707–1716 (2007).
Mu, X. et al. DEHP exposure impairs mouse oocyte cyst breakdown and primordial follicle assembly through estrogen receptor-dependent and independent mechanisms. J. Hazard. Mater. 298, 232–240 (2015).
Ahn, H. et al. Parabens inhibit the early phase of folliculogenesis and steroidogenesis in the ovaries of neonatal rats. Mol. Reprod. Dev. 79, 626–636 (2012).
Zhang, T. et al. Di-(2-ethylhexyl) phthalate and bisphenol A exposure impairs mouse primordial follicle assembly in vitro. Environ. Mol. Mutagen. 55, 343–353 (2014).
Zhou, C., Wang, W., Peretz, J. & Flaws, J. A. Bisphenol A exposure inhibits germ cell nest breakdown by reducing apoptosis in cultured neonatal mouse ovaries. Reprod. Toxicol. 57, 87–99 (2015).
Lea, R. G. et al. The fetal ovary exhibits temporal sensitivity to a 'real-life' mixture of environmental chemicals. Sci. Rep. 6, 22279 (2016). This extensive study on sheep showed that long-term exposure to sewage sludge containing environmental chemicals during early, mid, late (in particular) or the whole gestational period disrupts fetal folliculogenesis.
Pepling, M. E. Follicular assembly: mechanisms of action. Reproduction 143, 139–149 (2012).
Billig, H., Furuta, I. & Hsueh, A. J. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology 133, 2204–2212 (1993).
Anderson, R. A. et al. Activation of the aryl hydrocarbon receptor by a component of cigarette smoke reduces germ cell proliferation in the human fetal ovary. Mol. Hum. Reprod. 20, 42–48 (2014).
Paredes, A. et al. Loss of synaptonemal complex protein-1, a synaptonemal complex protein, contributes to the initiation of follicular assembly in the developing rat ovary. Endocrinology 146, 5267–5277 (2005).
Santamaría, C., Durando, M., Muñoz de Toro, M., Luque, E. H. & Rodriguez, H. A. Ovarian dysfunctions in adult female rat offspring born to mothers perinatally exposed to low doses of bisphenol A. J. Steroid Biochem. Mol. Biol. 158, 220–230 (2016).
Gámez, J. M. et al. Exposure to a low dose of bisphenol A impairs pituitary-ovarian axis in prepubertal rats. Effects on early folliculogenesis. Environ. Toxicol. Pharmacol. 39, 9–15 (2015).
Zheng, W. et al. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 23, 920–928 (2014).
Hannon, P. R., Brannick, K. E., Wang, W. & Flaws, J. A. Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol. Reprod. 92, 120 (2015). This study used cultured mouse ovaries to show that DEHP has no clear effect on the number of follicles, whereas exposure to its metabolite MEHP can reduce the number of germ cells, increase the number of primary follicles and induce a shift in expression of key factors favouring follicle recruitment.
Reddy, P. et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319, 611–613 (2008).
Rivera, O. E., Varayoud, J., Rodríguez, H. A., Muñoz-de-Toro, M. & Luque, E. H. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod. Toxicol. 32, 304–312 (2011).
Skakkebaek, N. E. et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol. Rev. 96, 55–97 (2016).
Woodruff, T. J., Schwartz, J. & Giudice, L. C. Research agenda for environmental reproductive health in the 21st century. J. Epidemiol. Community Health 64, 307–310 (2010).
Woodruff, T. J. Bridging epidemiology and model organisms to increase understanding of endocrine disrupting chemicals and human health effects. J. Steroid Biochem. Mol. Biol. 127, 108–117 (2011).
Menken, J., Trussell, J. & Larsen, U. Age and infertility. Science 233, 1389–1394 (1986).
Blomberg Jensen, M., Priskorn, L., Jensen, T. K., Juul, A. & Skakkebaek, N. E. Temporal trends in fertility rates: a nationwide registry based study from 1901 to 2014. PLoS ONE 10, e0143722 (2015).
Nelson, S. M., Telfer, E. E. & Anderson, R. A. The ageing ovary and uterus: new biological insights. Hum. Reprod. Update 19, 67–83 (2013).
Broekmans, F. J., Soules, M. R. & Fauser, B. C. Ovarian aging: mechanisms and clinical consequences. Endocr. Rev. 30, 465–493 (2009).
Cohn, B. A. et al. DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet 361, 2205–2206 (2003).
Fei, C., McLaughlin, J. K., Lipworth, L. & Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 24, 1200–1205 (2009).
Hanke, W. & Jurewicz, J. The risk of adverse reproductive and developmental disorders due to occupational pesticide exposure: an overview of current epidemiological evidence. Int. J. Occup. Med. Environ. Health 17, 223–243 (2004).
Hanson, B. et al. Female infertility, infertility-associated diagnoses, and comorbidities: a review. J. Assist. Reprod. Genet. 34, 167–177 (2017).
Cirillo, P. M., Wang, E. T., Cedars, M. I., Chen, L. & Cohn, B. A. Irregular menses predicts ovarian cancer: prospective evidence from the Child Health and Development Studies. Int. J. Cancer 139, 1009–1017 (2016).
Shaaban, A. M. et al. Ovarian malignant germ cell tumors: cellular classification and clinical and imaging features. Radiographics 34, 777–801 (2014).
Kraggerud, S. M. et al. Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr. Rev. 34, 339–376 (2013).
Ono, M. & Harley, V. R. Disorders of sex development: new genes, new concepts. Nat. Rev. Endocrinol. 9, 79–91 (2013).
Hughes, I. A., Houk, C., Ahmed, S. F., Lee, P. A. & Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology Consensus Group. Consensus statement on management of intersex disorders. J. Pediatr. Urol. 2, 148–162 (2006).
Pleskacova, J. et al. Tumor risk in disorders of sex development. Sex. Dev. 4, 259–269 (2010).
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal 13, 3978 (2015).
Christensen, K. L. Y., Makris, S. L. & Lorber, M. Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment. Regul. Toxicol. Pharmacol. 69, 380–389 (2014).
Liew, Z., Ritz, B., Virk, J. & Olsen, J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: a Danish national birth cohort study. Autism Res. 9, 951–958 (2016).
Ersbøll, A. S. et al. Changes in the pattern of paracetamol use in the periconception period in a Danish cohort. Acta Obstet. Gynecol. Scand. 94, 898–903 (2015).
Dierkes, G. et al. N-acetyl-4-aminophenol (paracetamol), N-acetyl-2-aminophenol and acetanilide in urine samples from the general population, individuals exposed to aniline and paracetamol users. Int. J. Hyg. Environ. Health 217, 592–599 (2014).
Nielsen, J. K. et al. N-acetyl-4-aminophenol (paracetamol) in urine samples of 6–11-year-old Danish school children and their mothers. Int. J. Hyg. Environ. Health. 218, 28–33 (2015).
Svingen, T. & Vinggaard, A. M. The risk of chemical cocktail effects and how to deal with the issue. J. Epidemiol. Community Health 70, 322–323 (2016).
Fowler, P. A. et al. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol. Hum. Reprod. 14, 269–280 (2008).
Bellingham, M. et al. Exposure to chemical cocktails before or after conception — the effect of timing on ovarian development. Mol. Cell. Endocrinol. 376, 156–172 (2013). This study revealed that a change in exposure between the pre-conception and post-conception periods affects fetal ovary development to a greater extent than does an unchanged, continuous exposure.
Hass, U. et al. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ. Health Perspect. 115, 122–128 (2007).
Christiansen, S. et al. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ. Health Perspect. 117, 1839–1846 (2009).
Tinwell, H. & Ashby, J. Sensitivity of the immature rat uterotrophic assay to mixtures of estrogens. Environ. Health Perspect. 112, 575–582 (2004).
van Meeuwen, J. A., van den Berg, M., Sanderson, J. T., Verhoef, A. & Piersma, A. H. Estrogenic effects of mixtures of phyto- and synthetic chemicals on uterine growth of prepubertal rats. Toxicol. Lett. 170, 165–176 (2007).
Charles, G. D. et al. Analysis of the interaction of phytoestrogens and synthetic chemicals: an in vitro/in vivo comparison. Toxicol. Appl. Pharmacol. 218, 280–288 (2007).
Jost, A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog. Horm. Res. 8, 379–418 (1953).
Acknowledgements
The authors thank the Ministry of Food and Environment of Denmark, who funded their work from 2011 to 2016.
Author information
Authors and Affiliations
Contributions
H.K.L.J., T.S. and J.B. researched data for the article, provided substantial contribution to discussion of content, wrote the article and reviewed and/or edited the manuscript before submission. P.A.F. and A.M.V. provided substantial contribution to discussion of content, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Endocrine-disrupting chemicals
-
The WHO defines an endocrine disruptor as “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)population”.
- Testicular dysgenesis syndrome
-
A collective term for male reproductive disorders (testicular germ cell cancer, cryptorchidism and some cases of poor sperm quality and hypospadias) that are hypothesized to share a common fetal origin; that is, disruptions of signalling pathways controlling early development of the male reproductive system.
- Cohesin complex
-
A multisubunit protein complex responsible for cohesion between sister chromatids during both mitosis and meiosis. It is also central to DNA double-strand break repair, especially in meiotic cells.
- Synaptonemal complex
-
A meiosis specific structure composed of multiple proteins that connect homologous chromosomes during prophase I.
- Nests
-
Clusters of germ cells connected by intercellular bridges.
- Disorders of sex development
-
Congenital conditions in which chromosomal, gonadal or anatomical sex is atypical.
Rights and permissions
About this article
Cite this article
Johansson, H., Svingen, T., Fowler, P. et al. Environmental influences on ovarian dysgenesis — developmental windows sensitive to chemical exposures. Nat Rev Endocrinol 13, 400–414 (2017). https://doi.org/10.1038/nrendo.2017.36
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2017.36
This article is cited by
-
Associations between lifestyle factors and levels of per- and polyfluoroalkyl substances (PFASs), phthalates and parabens in follicular fluid in women undergoing fertility treatment
Journal of Exposure Science & Environmental Epidemiology (2023)
-
Occupational differences in personal care product use and urinary concentration of endocrine disrupting chemicals by gender
Journal of Exposure Science & Environmental Epidemiology (2023)
-
Unlocking India’s Potential in Managing Endocrine-Disrupting Chemicals (EDCs): Importance, Challenges, and Opportunities
Exposure and Health (2023)
-
Transcriptional profiling of the developing rat ovary following intrauterine exposure to the endocrine disruptors diethylstilbestrol and ketoconazole
Archives of Toxicology (2023)
-
Dysfunctional Ovarian Stem Cells Due to Neonatal Endocrine Disruption Result in PCOS and Ovarian Insufficiency in Adult Mice
Stem Cell Reviews and Reports (2022)