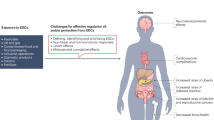
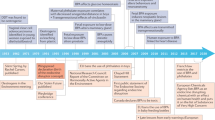
Abstract
Environmental endocrine disrupting chemicals (EDCs), including pesticides and industrial chemicals, have been and are released into the environment producing deleterious effects on wildlife and humans. The effects observed in animal models after exposure during organogenesis correlate positively with an increased incidence of malformations of the male genital tract and of neoplasms and with the decreased sperm quality observed in European and US populations. Exposure to EDCs generates additional effects, such as alterations in male and female reproduction and changes in neuroendocrinology, behavior, metabolism and obesity, prostate cancer and thyroid and cardiovascular endocrinology. This Review highlights the carcinogenic properties of EDCs, with a special focus on bisphenol A. However, humans and wildlife are exposed to a mixture of EDCs that act contextually. To explain this mindboggling complexity will require the design of novel experimental approaches that integrate the effects of different doses of structurally different chemicals that act at different ages on different target tissues. The key to this complex problem lies in the adoption of mathematical modeling and computer simulations afforded by system biology approaches. Regardless, the data already amassed highlight the need for a public policy to reduce exposure to EDCs.
Key Points
-
The embryo is an open system and the environment is a co-determinant of phenotypes, such that the embryo 'reads' environmental cues as a forecast of the postnatal environment
-
Hormones act as morphogens: extemporaneous exposure to even low doses of hormonally active chemicals increases the susceptibility to various diseases, including cancer
-
Neoplasia is a tissue-based disease caused by various deleterious exposures that interfere with the reciprocal communication between cells and between cells and their surrounding extracellular matrix
-
The effects of developmental exposure to diethylstilbestrol observed in humans have been reproduced in rodent models; thus, rodents are relevant models for assessing the human toxicity of environmental endocrine disruptors
-
Endocrine disrupting chemicals act additively—their multiple and complex effects are dose-dependent and contextual; therefore, a systems biology approach should be adopted to tackle this complexity
-
Sufficient supporting data have been gathered on the deleterious effects of endocrine disrupting chemicals to warrant immediate action to decrease human and wildlife exposure to these agents
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wingspread consensus statement in Chemically Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection (eds Colborn, T. & Clement, C.) 1–8 (Princeton Scientific Publishing, Princeton, 1992).
Colborn, T., vom Saal, F. S. & Soto, A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 101, 378–384 (1993).
Sharpe, R. M. & Skakkebaek, N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 341, 1392–1395 (1993).
Davis, D. L. et al. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ. Health Perspect. 101, 372–377 (1993).
Markey, C. M., Rubin, B. S., Soto, A. M. & Sonnenschein, C. Endocrine disruptors: from Wingspread to environmental developmental biology. J. Steroid Biochem. Mol. Biol. 83, 235–244 (2002).
vom Saal, F. S. et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 24, 131–138 (2007).
Crain, D. A. et al. Cellular bioavailability of natural hormones and environmental contaminants as a function of serum and cytosolic binding factors. Toxicol. Ind. Health 14, 261–273 (1998).
Diamanti-Kandarakis, E. et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342 (2009).
Kavlock, R. J. et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U. S. EPA-sponsored workshop. Environ. Health Perspect. 104 (Suppl. 4), 715–740 (1996).
Huggins, C. Endocrine-induced regression of cancers. Science 156, 1050–1054 (1967).
Mori, T., Bern, H. A., Mills, K. T. & Young, P. N. Long-term effects of neonatal steroid exposure on mammary gland development and tumorigenesis in mice. J. Natl Cancer Inst. 57, 1057–1061 (1976).
Lacassagne, A. Endocrine factors concerned in the genesis of experimental mammary carcinoma. J. Endocrinol. 13, ix–xviii (1955).
Huggins, C. Endocrine-induced regression of cancers. Cancer Res. 27, 1925–1930 (1967).
Gilbert, S. F., Opitz, J. M. & Raff, R. A. Resynthesizing evolutionary and developmental biology. Dev. Biol. 173, 357–372 (1996).
Griffiths, P. E. & Gray, R. D. in Cycles of Contingency: Developmental Systems and Evolution (eds Oyama, S., Griffiths, P. E. & Gray, R. D.) 195–218 (MIT Press, Cambridge, 2000).
Sonnenschein, C. & Soto, A. M. The Society of Cells: Cancer and Control of Cell Proliferation (Springer Verlag, New York, 1999).
Baker, S. G. & Kramer, B. S. Paradoxes in carcinogenesis: new opportunities for research directions. BMC Cancer 7, 151 (2007).
Phillips, R. B. Adaptive evolution or genetic drift? Does genome complexity produce organismal complexity? Heredity 93, 122–123 (2004).
Moss, L. What Genes Can't Do (MIT Press, Cambridge, MA, 2003).
Hull, D. The Philosophy of Biological Science (Prentice Hall, Englewood Clifts, NJ, 1974).
Gilbert, S. F. & Sarkar, S. Embracing complexity: organicism for the 21st century. Dev. Dyn. 219, 1–9 (2000).
Noble, D. The Music of Life: Biology beyond the Genome (Oxford University Press, Oxford, 2006).
Ritter, W. E. & Bailey, E. W. The organismal conception: its place in science and its bearing on philosophy. Univ. Calif. Pub. Zool. 31, 307–358 (1928).
Gilbert, S. F. & Epel, D. Ecological Developmental Biology (Sinauer Associates, Sunderland, MA, 2009).
Gilbert, S. F. Mechanisms for the environmental regulation of gene expression: ecological aspects of animal development. J. Biosci. 30, 65–74 (2005).
Barker, D. J., Eriksson, J. G., Forsén, T. & Osmond, C. Fetal origins of adult disease: strength of effects and biological basis. Int. J. Epidemiol. 31, 1235–1239 (2002).
Soto, A. M., Sonnenschein, C. & Miquel, P. A. On physicalism and downward causation in developmental and cancer biology. Acta Biotheor. 56, 257–274 (2008).
Weinberg, R. A. One Renegade Cell: How Cancer Begins (Basic Books, New York, 1998).
Ho, S.-M., Tang, W. Y., Belmonte de Frausto, J. & Prins, G. S. Developmental exposure to estradiol and bisphenol a increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 66, 5624–5632 (2006).
Keri, R. A. et al. An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod. Toxicol. 24, 240–252 (2007).
Soto, A. M. & Sonnenschein, C. The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays 26, 1097–1107 (2004).
Cooper, M. Regenerative pathologies: stem cells, teratomas and theories of cancer. Medicine Studies 1, 55–66 (2009).
Baker, S. G. et al. Plausibility of stromal initiation of epithelial cancers without a mutation in the epithelium: a computer simulation of morphostats. BMC Cancer 9, 89–99 (2009).
Sonnenschein, C. & Soto, A. M. Theories of carcinogenesis: an emerging perspective. Semin. Cancer Biol. 18, 372–377 (2008).
Zarbl, H., Sukumar, S., Arthur, A. V., Martin-Zanca, D. & Barbacid, M. Direct mutagenesis of Ha-ras-1 oncogenes by n-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. Nature 315, 382–385 (1985).
Maffini, M. V., Soto, A. M., Calabro, J. M., Ucci, A. A. & Sonnenschein, C. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 117, 1495–1502 (2004).
Barcellos-Hoff, M. H. & Ravani, S. A. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 60, 1254–1260 (2000).
Maffini, M. V., Calabro, J. M., Soto, A. M. & Sonnenschein, C. Stromal regulation of neoplastic development: Age-dependent normalization of neoplastic mammary cells by mammary stroma. Am. J. Pathol. 67, 1405–1410 (2005).
Hendrix, M. J. et al. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer 7, 246–255 (2007).
Coleman, W., Wennerberg, A. E., Smith, G. J. & Grisham, J. W. Regulation of the differentiation of diploid and aneuploid rat liver epithelial (stemlike) cells by the liver microenvironment. Am. J. Pathol. 142, 1373–1382 (1993).
Mintz, B. & Ilmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl Acad. Sci. USA 72, 3585–3589 (1975).
vom Saal, F. S. & Bronson, F. H. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science 208, 597–599 (1980).
Vandenberg, L. N. et al. Exposure to the xenoestrogen bisphenol-A alters development of the fetal mammary gland. Endocrinology 148, 116–127 (2007).
vom Saal, F. S., Grant, W. M., McMullen, C. W. & Laves, K. S. High fetal estrogen concentrations: correlation with increased adult sexual activity and decreased aggression in male mice. Science 220, 1306–1309 (1983).
Markey, C. M., Michaelson, C. L., Veson, E. C., Sonnenschein, C. & Soto, A. M. The rodent uterotrophic assay: response to Ashby and Newbold et al. Environ. Health Perspect. 109, A569–A570 (2001).
Markey, C. M., Wadia, P. R., Rubin, B. S., Sonnenschein, C. & Soto, A. M. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol. Reprod. 72, 1344–1351 (2005).
Mittendorf, R. Teratogen update: carcinogenesis and teratogenesis associated with exposure to diethylstilbestrol (DES) in utero. Teratology 51, 435–445 (1995).
Kurita, T., Mills, A. & Cunha, G. R. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development 131, 1639–1649 (2004).
Newbold, R. R., Moore, A. B. & Dixon, D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES). Toxicol. Pathol. 30, 611–616 (2002).
Burroughs, K. D., Fuchs-Young, R., Davis, R. & Walker, C. L. Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol. Reprod. 63, 1322–1330 (2000).
Miller, C., Degenhardt, K. & Sassoon, D. A. Fetal exposure to DES results in de-regulation of Wnt7a during uterine morphogenesis. Nat. Genet. 20, 228–230 (1998).
Baird, D. D. & Newbold, R. Prenatal diethylstilbestrol (DES) exposure is associated with uterine leiomyoma development. Reprod. Toxicol. 20, 81–84 (2005).
Rothschild, T. C., Boylan, E. S., Calhoon, R. E. & Vonderhaar, B. K. Transplacental effects of diethylstilbestrol on mammary development and tumorigenesis in female ACI rats. Cancer Res. 47, 4508–4516 (1987).
Boylan, E. S. & Calhoon, R. E. Mammary tumorigenesis in the rat following prenatal exposure to diethylstilbestrol and postnatal treatment with 7, 12-dimethylbenz[a]anthracene. J. Toxicol. Environ. Health 5, 1059–1071 (1979).
Palmer, J. R. et al. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 15, 1509–1514 (2006).
Trichopoulos, D. Is breast cancer initiated in utero? Epidemiology 1, 95–96 (1990).
Braun, M. M., Ahlbom, A., Floderus, B., Brinton, L. A. & Hoover, R. N. Effect of twinship on incidence of cancer of the testis, breast, and other sites (Sweden). Cancer Causes Control 6, 519–524 (1995).
Potischman, N. & Troisi, R. In-utero and early life exposures in relation to risk of breast cancer. Cancer Causes Control 10, 561–573 (1999).
Halakivi-Clarke, L., Cho, E., Onojafe, I., Liao, D. J. & Clarke, R. Maternal exposure to tamoxifen during pregnancy increases carcinogen-induced mammary tumorigenesis among female rat offspring. Clin. Cancer Res. 6, 305–308 (2000).
Calafat, A. M., Ye, X., Wong, L. Y., Reidy, J. A. & Needham, L. L. Exposure of the U. S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 116, 39–44 (2008).
Schönfelder, G. et al. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 110, A703–A707 (2002).
Sun, Y. et al. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 18, 501–507 (2004).
Wong, K. O., Leo, L. W. & Seah, H. L. Dietary exposure assessment of infants to bisphenol A from the use of polycarbonate baby milk bottles. Food Addit. Contam. 22, 280–288 (2005).
Durando, M. et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ. Health Perspect. 115, 80–86 (2007).
Murray, T. J., Maffini, M. V., Ucci, A. A., Sonnenschein, C. & Soto, A. M. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod. Toxicol. 23, 383–390 (2007).
Jenkins, S. et al. Oral exposure to bisphenol A increases dimethylbenzanthracene-induced mammary cancer in rats. Environ. Health Perspect. 117, 910–915 (2009).
Muñoz-de-Toro, M. M. et al. Perinatal exposure to bisphenol A alters peripubertal mammary gland development in mice. Endocrinology 146, 4138–4147 (2005).
Vandenberg, L. N. et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod. Toxicol. 26, 210–219 (2008).
Hatch, E. E. et al. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA 280, 630–634 (1998).
Couse, J. F. et al. Accelerated onset of uterine tumors in transgenic mice with aberrant expression of the estrogen receptor after neonatal exposure to diethylstilbestrol. Mol. Carcinog. 19, 236–242 (1997).
Steenland, K., Bertazzi, P., Baccarelli, A. & Kogevinas, M. Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environ. Health Perspect. 112, 1265–1268 (2004).
Kogevinas, M. Human health effects of dioxins: cancer, reproductive and endocrine system effects. Hum. Reprod. Update 7, 331–339 (2001).
Buchanan, D. L., Sato, T., Peterson, R. E. & Cooke, P. S. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse uterus: critical role of the aryl hydrocarbon receptor in stromal tissue. Toxicol. Sci. 57, 302–311 (2000).
Fenton, S. E., Hamm, J. T., Birnbaum, L. & Youngblood, G. L. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Sci. 67, 63–74 (2002).
Brown, N. M., Manzolillo, P. A., Zhang, J. X., Wang, J. & Lamartiniere, C. A. Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis 19, 1623–1629 (1998).
Høyer, A. P., Grandjean, P., Jørgensen, T., Brock, J. W. & Hartvig, H. B. Organochlorine exposure and risk of breast cancer. Lancet 352, 1816–1820 (1998).
Cohn, B. A., Wolff, M. S., Cirillo, P. M. & Sholtz, R. I. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ. Health Perspect. 115, 1406–1414 (2007).
Ibarluzea, J. M. et al. Breast cancer risk in the combined effect of environmental estrogens. Cancer Causes Control 15, 591–600 (2004).
Skakkebaek, N. E., Rajpert- De Meyts, E. & Main, K. M. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 16, 972–978 (2001).
Hardell, L., Bavel, B., Lindstrom, G., Eriksson, M. & Carlberg, M. In utero exposure to persistent organic pollutants in relation to testicular cancer risk. Int. J. Androl. 29, 228–234 (2006).
Timms, B. G. et al. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl Acad. Sci. USA 102, 7014–7019 (2005).
Timms, B. G., Peterson, R. E. & vom Saal, F. S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin interacts with endogenous estradiol to disrupt prostate gland morphogenesis in male rat fetuses. Toxicol. Sci. 67, 264–274 (2002).
Bosland, M. C. et al. Multistage prostate carcinogenesis: the role of hormones. Princess Takamatsu Symp. 22, 109–123 (1991).
Huang, L., Pu, Y., Alam, S., Birch, L. & Prins, G. S. Estrogenic regulation of signaling pathways and homeobox genes during rat prostate development. J. Androl. 25, 330–337 (2004).
Prins, G. S., Birch, L., Tang, W.-Y. & Ho, S.-M. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod. Toxicol. 23, 374–382 (2007).
Crain, D. A. et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 90, 911–940 (2008).
Howdeshell, K. L., Hotchkiss, A. K., Thayer, K. A., Vandenbergh, J. G. & vom Saal, F. S. Exposure to bisphenol A advances puberty. Nature 401, 763–764 (1999).
Newbold, R. R., Padilla-Banks, E., Jefferson, W. N. & Heindel, J. J. Effects of endocrine disruptors on obesity. Int. J. Androl. 31, 201–208 (2008).
Rubin, B. S., Murray, M. K., Damassa, D. A., King, J. C. & Soto, A. M. Perinatal exposure to low doses of bisphenol-A affects body weight, patterns of estrous cyclicity and plasma LH levels. Environ. Health Perspect. 109, 675–680 (2001).
Nunez, A. A., Kannan, K., Giesy, J. P., Fang, J. & Clemens, L. G. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 42, 917–922 (2001).
Alonso-Magdalena, P., Morimoto, S., Ripoll, C., Fuentes, E. & Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 114, 106–112 (2006).
Kortenkamp, A. Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. Int. J. Androl. 29, 193–198 (2006).
Moriyama, K. et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 87, 5185–5190 (2002).
Noble, D. From the Hodgkin-Huxley axon to the virtual heart. J. Physiol. 580, 15–22 (2007).
Acknowledgements
This work was supported by grants from the Parsemus Foundation and the NIH (ES0150182, ES012301, ES08314 and ES018822). We are grateful to Cheryl Schaeberle and Michael Askenase for their excellent editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Soto, A., Sonnenschein, C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol 6, 363–370 (2010). https://doi.org/10.1038/nrendo.2010.87
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2010.87
This article is cited by
-
Presence of pharmaceuticals and bacterial resistance genes in river epilithic biofilms exposed to intense agricultural and urban pressure
Environmental Monitoring and Assessment (2023)
-
Endocrine-disrupting chemicals (EDCs) and cancer: new perspectives on an old relationship
Journal of Endocrinological Investigation (2022)
-
Bisphenol A and cancer: a prelude to challenging epidemiology
International Archives of Occupational and Environmental Health (2022)
-
Impact of Endocrine Disrupting Chemicals (EDCs) on Reproductive Health of Human
Proceedings of the Zoological Society (2022)
-
Virtual screening of potentially endocrine-disrupting chemicals against nuclear receptors and its application to identify PPARγ-bound fatty acids
Archives of Toxicology (2021)