Abstract
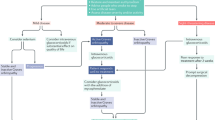
Advances in the past few years have helped clinicians understand some of the pathogenetic mechanisms of Graves orbitopathy (GO), particularly the role of receptors for TSH and insulin-like growth factor I in the orbit. Optimal treatment strategies have been formulated and published by the European Group on Graves' Orbitopathy, which are hoped to improve the management of patients with this condition. The administration of intravenous pulses of steroids has been established as a superior treatment approach compared with other steroid regimens. In addition, orbital radiotherapy was effective in a subgroup of patients with GO who had eye dysmotility. The use of immunotherapies for the treatment of GO is currently being explored; of these, rituximab has emerged as a promising new agent.
Key Points
-
Graves orbitopathy is an autoimmune disease that is closely associated with Graves disease
-
Smoking, hypothyroidism and radioiodine therapy can exacerbate Graves orbitopathy
-
Immunosuppressive therapy is only effective during the initial, active phase of the disease
-
High doses of steroids administered as intravenous pulses are more effective and associated with fewer adverse effects than orally administered steroids
-
Surgical decompression of the orbit has a major role in management of patients with Graves orbitopathy complicated by optic neuropathy who fail to respond to medical treatment
-
Rehabilitative, reconstructive surgery used in the inactive phase of the disease has an important role in restoration of the function and appearance of the eye
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wiersinga, W. M. Management of Graves' ophthalmopathy. Nat. Clin. Pract. Endocrinol. Metab. 3, 396–404 (2007).
Coulter, I. et al. Psychological implications of Graves' orbitopathy. Eur. J. Endocrinol. 157, 127–131 (2007).
Salvi, M. et al. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunological abnormalities. J. Clin. Endocrinol. Metab. 70, 89–94 (1990).
Khoo, D. H. et al. Graves' ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid 10, 1093–1100 (2000).
Orgiazzi, J. in Graves' Orbitopathy. A Multidisciplinary Approach (eds Wiersinga, W. M. & Kahaly, G. J.) 41–56 (Karger Publishers, Basel, 2007).
Perros, P. & Kendall-Taylor, P. Biological activity of autoantibodies from patients with thyroid-associated ophthalmopathy: in vitro effects on porcine extraocular myoblasts. Q. J. Med. 84, 691–706 (1992).
Perros, P. & Kendall-Taylor, P. Demonstration of thyrotropin binding sites in orbital connective tissue: possible role in the pathogenesis of thyroid-associated ophthalmopathy. J. Endocrinol. Invest. 17, 163–170 (1994).
Valyasevi, R. W. et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J. Clin. Endocrinol. Metab. 84, 2557–2562 (1999).
Wakelkamp, I. M. et al. TSH-R expression and cytokine profile in orbital tissue of active vs inactive Graves' ophthalmopathy patients. Clin. Endocrinol. (Oxf.) 58, 280–287 (2003).
Boschi, A. et al. Quantification of cells expressing the thyrotropin receptor in extraocular muscles in thyroid associated orbitopathy. Br. J. Ophthalmol. 89, 724–729 (2005).
Agretti, P. et al. Evidence for protein and mRNA TSHR expression in fibroblasts from patients with thyroid-associated ophthalmopathy (TAO) after adipocytic differentiation. Eur. J. Endocrinol. 152, 777–784 (2005).
Gerding, M. N. et al. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin. Endocrinol. (Oxf.) 52, 267–271 (2000).
Weightman, D. R. et al. Autoantibodies to IGF-I binding sites in thyroid associated ophthalmopathy. Autoimmunity 16, 251–257 (1993).
Douglas, R. S. et al. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. J. Immunol. 178, 3281–3287 (2007).
Smith, T. J. et al. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid 18, 983–988 (2008).
Bednarczuk, T. et al. Susceptibility genes in Graves' ophthalmopathy: searching for a needle in a haystack? Clin. Endocrinol. (Oxf.) 67, 3–19 (2007).
Jacobson, E. M. & Tomer, Y. The genetic basis of thyroid autoimmunity. Thyroid 17, 949–961 (2007).
Perros, P. et al. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid–eye clinic. Clin. Endocrinol. (Oxf.) 38, 367–372 (1993).
Wiersinga, W. M. Thyroid-associated ophthalmopathy: pediatric and endocrine aspects. Pediatr. Endocrinol. Rev. 1 (Suppl. 3), 513–517 (2004).
Krassas, G. E. et al. Childhood Graves' ophthalmopathy: results of a European questionnaire study. Eur. J. Endocrinol. 153, 515–521 (2005).
Krassas, G. E. & Wiersinga, W. Smoking and autoimmune thyroid disease: the plot thickens. Eur. J. Endocrinol. 154, 777–780 (2006).
Pfeilschifter, J. & Ziegler, R. Smoking and endocrine ophthalmopathy: impact of smoking severity and current vs lifetime cigarette consumption. Clin. Endocrinol. (Oxf.) 45, 477–481 (1996).
Cawood, T. J. et al. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J. Clin. Endocrinol. Metab. 92, 59–64 (2007).
Dickinson, A. J. & Perros, P. Controversies in the clinical evaluation of active thyroid-associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin. Endocrinol. (Oxf.) 55, 283–303 (2001).
Wiersinga, W. M. et al. Clinical assessment of patients with Graves' orbitopathy: the European Group on Graves' Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur. J. Endocrinol. 155, 387–389 (2006).
Bartley, G. B. & Gorman, C. A. Diagnostic criteria for Graves' ophthalmopathy. Am. J. Ophthalmol. 119, 792–795 (1995).
McKeag, D. et al. Clinical features of dysthyroid optic neuropathy: a European Group on Graves' Orbitopathy (EUGOGO) survey. Br. J. Ophthalmol. 91, 455–458 (2007).
Hales, I. B. & Rundle, F. F. Ocular changes in Graves' disease. A long-term follow-up study. Q. J. Med. 29, 113–126 (1960).
Baldeschi, L. et al. Reactivation of Graves' orbitopathy after rehabilitative orbital decompression. Ophthalmology 114, 1395–1402 (2007).
Bartalena, L. et al. Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Eur. J. Endocrinol. 58, 273–285 (2008).
Perros, P. & Kendall-Taylor, P. Medical treatment for thyroid-associated ophthalmopathy. Thyroid 12, 241–244 (2002).
Bartalena, L. et al. Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N. Engl. J. Med. 338, 73–78 (1998).
Tallstedt, L. et al. Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. N. Engl. J. Med. 326, 1733–1738 (1992).
Bartalena, L. et al. Glucocorticoids and outcome of radioactive iodine therapy for Graves' hyperthyroidism. Eur. J. Endocrinol. 153, 13–14 (2005).
Perros, P. et al. A prospective study of the effects of radioiodine therapy for hyperthyroidism in patients with minimally active Graves' ophthalmopathy. J. Clin. Endocrinol. Metab. 90, 5321–5323 (2005).
Järhult, J. et al. Graves' disease with moderate–severe endocrine ophthalmopathy—long-term results of a prospective, randomized study of total or subtotal thyroid resection. Thyroid 15, 1157–1164 (2005).
Menconi, F. et al. Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves' orbitopathy treated with intravenous glucocorticoids. J. Clin. Endocrinol. Metab. 92, 1653–1658 (2007).
Hart, R. H. & Perros, P. Glucocorticoids in the medical management of Graves' ophthalmopathy. Minerva Endocrinol. 28, 223–231 (2003).
Kahaly, G. J. et al. Randomized, single-blind trial of intravenous versus oral steroid monotherapy in Graves' orbitopathy. J. Clin. Endocrinol. Metab. 90, 5234–5240 (2005).
Marcocci, C. et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves' ophthalmopathy: results of a prospective, single-blind, randomized study. J. Clin. Endocrinol. Metab. 86, 3562–3567 (2001).
Hart, R. H. et al. Early response to intravenous glucocorticoids for severe thyroid-associated ophthalmopathy predicts treatment outcome. J. Ocul. Pharmacol. Ther. 21, 328–336 (2005).
Le Moli, R. et al. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves' ophthalmopathy. Thyroid 17, 357–362 (2007).
Perros, P. et al. A questionnaire survey on the management of Graves' orbitopathy in Europe. Eur. J. Endocrinol. 155, 207–211 (2006).
Ramos, H. E. et al. Management of Graves' orbitopathy in Latin America: an international questionnaire study compared with Europe. Clin. Endocrinol. (Oxf.) 69, 951–956 (2008).
Bradley, E. A. et al. Orbital radiation for Graves ophthalmopathy: a report by the American Academy of Ophthalmology. Ophthalmology 115, 398–409 (2008).
Wakelkamp, I. M. et al. Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves' ophthalmopathy? A randomized controlled trial. Clin. Endocrinol. (Oxf.) 63, 323–328 (2005).
Mourits, M. P. et al. Radiotherapy for Graves' orbitopathy: randomised placebo-controlled study. Lancet 355, 1505–1509 (2000).
Prummel, M. F. et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves' ophthalmopathy. J. Clin. Endocrinol. Metab. 89, 15–20 (2004).
Baldeschi, L. in Graves' Orbitopathy. A Multidisciplinary Approach (eds Wiersinga, W. M. & Kahaly, G. J.) 160–175 (Karger Publishers, Basel, 2007).
Neoh, C. & Eckstein, A. in Graves' Orbitopathy. A Multidisciplinary Approach, (eds Wiersinga, W. M. & Kahaly, G. J) 188–200 (Karger Publishers, Basel, 2007).
Finamor, F. E. et al. Pentoxifylline (PTX)-—an alternative treatment in Graves' ophthalmopathy (inactive phase): assessment by a disease-specific quality of life questionnaire and by exophthalmometry in a prospective randomized trial. Eur. J. Ophthalmol. 14, 277–283 (2004).
Bartalena, L. et al. Graves' ophthalmopathy: state of the art and perspectives. J. Endocrinol. Invest. 27, 295–301 (2004).
Bouzas, E. A. et al. Antioxidant agents in the treatment of Graves' ophthalmopathy. Am. J. Ophthalmol. 129, 618–622 (2000).
Dickinson, A. J. et al. Double-blind, placebo-controlled trial of octreotide long-acting repeatable (LAR) in thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 89, 5910–5915 (2004).
Wémeau, J. L. et al. Octreotide (long-acting release formulation) treatment in patients with Graves' orbitopathy: clinical results of a four-month, randomized, placebo-controlled, double-blind study. J. Clin. Endocrinol. Metab. 90, 841–848 (2005).
Chang, T. C. & Liao, S. L. Slow-release lanreotide in Graves' ophthalmopathy: a double-blind randomized, placebo-controlled clinical trial. J. Endocrinol. Invest. 29, 413–422 (2006).
Stan, M. N. et al. Randomized, double-blind, placebo-controlled trial of long-acting release octreotide for treatment of Graves' ophthalmopathy. J. Clin. Endocrinol. Metab. 91, 4817–4824 (2006).
El Fassi, D. et al. Treatment-resistant severe, active Graves' ophthalmopathy successfully treated with B lymphocyte depletion. Thyroid 16, 709–710 (2006).
Salvi, M. et al. Treatment of Graves' disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: an open study. Eur. J. Endocrinol. 156, 33–40 (2007).
Nielsen, C. H. et al. B-cell depletion with rituximab in the treatment of autoimmune diseases. Graves' ophthalmopathy: the latest addition to an expanding family. Expert Opin. Biol. Ther. 7, 1061–1078 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Perros, P., Krassas, G. Graves orbitopathy: a perspective. Nat Rev Endocrinol 5, 312–318 (2009). https://doi.org/10.1038/nrendo.2009.61
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.61