Abstract
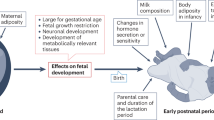
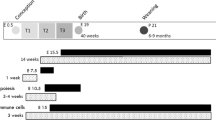
The primary markers of the metabolic syndrome are central obesity, insulin resistance and hypertension. In this review, we consider the effect of changes in maternal nutrition during critical windows in fetal development on an individual's subsequent predisposition to the metabolic syndrome. The fetal origins of obesity, cardiovascular disease and insulin resistance have been investigated in a wide range of epidemiological and animal studies; these investigations highlight adaptations made by the nutritionally manipulated fetus that aim to maintain energy homeostasis to ensure survival. One consequence of such developmental plasticity may be a long term re-setting of cellular energy homeostasis, most probably via epigenetic modification of genes involved in a number of key regulatory pathways. For example, reduced maternal–fetal nutrition during early gestation to midgestation affects adipose tissue development and adiposity of the fetus by setting an increased number of adipocyte precursor cells. Importantly, clinically relevant adaptations to nutritional challenges in utero may only manifest as primary components of the metabolic syndrome if followed by a period of accelerated growth early in the postnatal period and/or if offspring become obese.
Key Points
-
Foundations for the metabolic syndrome may be laid down in very early life as a consequence of changes in dietary supply to the rapidly growing conceptus and/or postnatal offspring
-
Critical windows of development, during which the fetus is susceptible to nutritional programming, must be considered in the etiology of the metabolic syndrome
-
A common epigenetic mechanism is fundamental to developmental programming of endocrine balance and cellular energy metabolism; the latter determines the partition of energy between catabolism and energy storage
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Björntorp, P. Metabolic implications of body fat distribution. Diabetes Care 14, 1132–1143 (1991).
Alberti, K. G. & Zimmet, P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
Hamaguchi, M. et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 143, 722–728 (2005).
Lemieux, I. et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler. Thromb. Vasc. Biol. 21, 961–967 (2001).
DeFronzo, R. A. & Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14, 173–194 (1991).
Ferrannini, E. Is insulin resistance the cause of the metabolic syndrome? Ann. Med. 38, 42–51 (2006).
Roberts, R. M. et al. Research priorities. Farm animal research in crisis. Science 324, 468–469 (2009).
Sattar, N. The metabolic syndrome: should current criteria influence clinical practice? Curr. Opin. Lipidol. 17, 404–411 (2006).
Poulsen, P., Vaag, A. A., Kyvik, K. O., Møller Jensen, D. & Beck-Nielson, H. Low birth weight is associated with NIDDM in monozygotic and dizygotic twin pairs. Diabetologia 40, 439–446 (1997).
Ferrannini, E. et al. Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR). Hypertension 30, 1144–1149 (1997).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001).
Zimmet, P. et al. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr. Diabetes 8, 299–306 (2007).
Lebovitz, H. E. & Banerji, M. A. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care 28, 2322–2325 (2005).
Després, J. P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 444, 881–887 (2006).
Abbasi, F. & Reaven, G. M. Relationship between fasting and day-long plasma glucose concentrations in diet-treated patients with type 2 diabetes. Metabolism 51, 457–459 (2002).
Seidell, J. C. Obesity, insulin resistance and diabetes--a worldwide epidemic. Br. J. Nutr. 83 (Suppl. 1), S5–S8 (2000).
Tantisira, K. G. et al. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am. J. Respir. Crit. Care Med. 178, 325–331 (2008).
Field, A. E., Cook, N. R. & Gillman, M. W. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes. Res. 13, 163–169 (2005).
Pan, Y. & Pratt, C. A. Metabolic syndrome and its association with diet and physical activity in US adolescents. J. Am. Diet Assoc. 108, 276–286 (2008).
Yajnik, C. S. Obesity epidemic in India intrauterine origins? Proc. Nutr. Soc. 63, 387–396 (2004).
Yajnik, C. S. et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int. J. Obes. Relat. Metab. Disord. 27, 173–180 (2003).
Kuo, L. E. et al. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann. NY Acad. Sci. 1148, 232–237 (2008).
Keith, S. W. et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int. J. Obes. (Lond.) 30, 1585–1594 (2006).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Symonds, M. E. Nutrition and its contribution to obesity and diabetes: a life-course approach to disease prevention? Proc. Nutr. Soc. 68, 71–77 (2009).
Symonds, M. E., Stephenson, T., Gardner, D. S. & Budge, H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod. Fertil. Dev. 19, 53–63 (2007).
Rao, S. et al. Maternal activity in relation to birth size in rural India. The Pune Maternal Nutrition Study. Eur. J. Clin. Nutr. 57, 531–542 (2003).
Mathews, F., Yudkin, P. & Neil, A. Influence of maternal nutrition on outcome of pregnancy: prospective cohort study. BMJ 319, 339–343 (1999).
Reynolds, R. M., Godfrey, K. M., Barker, M., Osmond, C. & Phillips, D. I. Stress responsiveness in adult life: Influence of mother's diet in late pregnancy. J. Clin. Endocrinol. Metab. 92, 2208–2210 (2007).
Barker, D. J., Bull, A. R., Osmond, C. & Simmonds, S. J. Fetal and placental size and risk of hypertension in adult life. BMJ 301, 259–262 (1990).
Pepper, G. V. and Craig Roberts, S. Rates of nausea and vomiting in pregnancy and dietary characteristics across populations. Proc. Biol. Sci. 273, 2675–2679 (2006).
Neville, M. C. Adaptation of maternal lipid flux to pregnancy: research needs. Eur. J. Clin. Nutr. 53 (Suppl. 1), S120–S123 (1999).
Symonds, M. E., Sebert, S. P. & Budge, H. The impact of diet during early life and its contribution to later disease—critical checkpoints in development and their long term consequences for metabolic health. Proc. Nutr. Soc. doi:10.1017/50029665109990152.
McCance, R. A. & Widdowson, E. M. The determinants of growth and form. Proc. R. Soc. Lond. B Biol. Sci. 185, 1–17 (1974).
Streeter, G. L. Weight, sitting height, head size, foot length and menstrual age of the human embryo. Contrib. Embryol. 11, 143 (1920).
Villar, J. & Belizan, J. M. The timing factor in the pathophysiology of intrauterine growth retardation syndrome. Obstet. Gynecol. Surv. 37, 449–506 (1982).
Fleischman, A. R., Oakes, G. K., Epstein, M. F., Catt, K. J. & Chez, R. A. Plasma renin activity during ovine pregnancy. Am. J. Physiol. 228, 901–904 (1975).
Ravelli, A. C. et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177 (1998).
Roseboom, T. J. et al. Effects of prenatal exposure to the Dutch famine on adult disease in later life: An overview. Twin Res. 4, 293–298 (2001).
Painter, R. C., Roseboom, T. J. & Bleker, O. P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 20, 345–352 (2005).
Lopuhaä, C. E. et al. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax 55, 555–561 (2000).
Painter, R. C. et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J. Am. Soc. Nephrol. 16, 189–194 (2005).
Roseboom, T. J. et al. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr. 72, 1101–1106 (2000).
Ravelli, A. C., Van der Meulen, J. H., Osmund, C., Barker, D. J. & Bleker, O. P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 70, 811–816 (1999).
Roseboom, T. J. et al. Coronary heart disease after prenatal exposure to the Dutch famine. Heart 84, 595–598 (2000).
Naeye, R. L. Do placental weights have clinical significance? Hum. Pathol. 18, 387–391 (1987).
Fowden, A. L., Li, J. & Forhead, A. J. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc. Nutr. Soc. 57, 113–122 (1998).
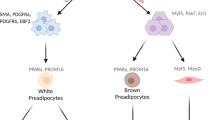
Symonds, M. E., Mostyn, A., Pearce, S., Budge, H. & Stephenson, T. Endocrine and nutritional regulation of fetal adipose tissue development. J. Endocrinol. 179, 293–299 (2003).
Bispham, J. et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 144, 3575–3585 (2003).
Gardner, D. S. et al. Programming of glucose-insulin metabolism in adult sheep after maternal undernutrition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R947–R954 (2005).
Costello, P. M. et al. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J. Physiol. 586, 2371–2379 (2008).
Wu, Z., Puigserver, P. & Spiegelman, B. M. Transcriptional activation of adipogenesis. Curr. Opin. Cell Biol. 11, 689–694 (1999).
Spalding, K. L. et al. Dynamics of fat cell turnover in humans. Nature 453, 783–787 (2008).
Harrington, T. A., Thomas, E. L., Frost, G., Modi, N. & Bell, J. D. Distribution of adipose tissue in the newborn. Pediatr. Res. 55, 437–441 (2004).
Modi, N. et al. Determinants of adiposity during preweaning postnatal growth in appropriately grown and growth-restricted term infants. Pediatr. Res. 60, 345–348 (2006).
Crowther, N. J., Cameron, N., Trusler, J. & Gray, I. P. Association between poor glucose tolerance and rapid post natal weight gain in seven-year-old children. Diabetologia 41, 1163–1167 (1998).
Iñiguez, G. et al. Longitudinal changes in insulin-like growth factor-I, insulin sensitivity, and secretion from birth to age three years in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 91, 4645–4649 (2006).
Ong, K. K. & Loos, R. J. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 95, 904–908 (2006).
Singhal, A. & Lucas, A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 363, 1642–1645 (2004).
Symonds, M. E. Integration of physiological and molecular mechanisms of the developmental origins of adult disease: new concepts and insights. Proc. Nutr. Soc. 66, 442–450 (2007).
McMillen, I. C. & Robinson, J. S. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633 (2005).
Snoeck, A., Remacle, C., Reusens, B. & Hoet, J. J. Effect of low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol. Neonate 57, 107–118 (1990).
Gopalakrishnan, G. S. et al. Influence of maternal pre-pregnancy body composition and diet during early-mid pregnancy on cardiovascular function and nephron number in juvenile sheep. Br. J. Nutr. 94, 938–947 (2005).
Gallou-Kabani, C., Vigé, A., Gross, M. S. & Junien, C. Nutri-epigenomics: lifelong remodelling of our epigenomes by nutritional and metabolic factors and beyond. Clin. Chem. Lab. Med. 45, 321–327 (2007).
Gallou-Kabani, C., Vigé, A. & Junien, C. Lifelong circadian and epigenetic drifts in metabolic syndrome. Epigenetics 2, 137–146 (2007).
Zeisel, S. H. Epigenetic mechanisms for nutrition determinants of later health outcomes. Am. J. Clin. Nutr. 89, S1488–S1493 (2009).
Malenfant, P. et al. Fat content in individual muscle fibers of lean and obese subjects. Int. J. Obes. Relat. Metab. Disord. 25, 1316–1321 (2001).
Sun, G., Ukkola, O., Rankinen, T., Joanisse, D. R. & Bouchard, C. Skeletal muscle characteristics predict body fat gain in response to overfeeding in never-obese young men. Metabolism 51, 451–456 (2002).
Casba, G. Phylogeny and ontogeny of hormone receptors: the selection theory of receptor formation and hormonal imprinting. Biol. Rev. Camb. Philos. Soc. 55, 47–63 (1980).
Nakae, J., Kido, Y. & Accili, D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev. 22, 818–835 (2001).
DeLellis, K. et al. IGF1 genotype, mean plasma level and breast cancer risk in the Hawaii/Los Angeles multiethnic cohort. Br. J. Cancer 88, 277–282 (2003).
Gluckman, P. D., Hanson, M. A. & Beedle, A. S. Non-genomic transgenerational inheritance of disease risk. Bioessays 29, 145–154 (2007).
Hales, C. N. & Barker, D. J. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35, 595–601 (1992).
Vickers, M. H., Ikenasio, B. A. & Breier, B. H. IGF-I treatment reduces hyperphagia, obesity, and hypertension in metabolic disorders induced by fetal programming. Endocrinology 142, 3964–3973 (2001).
Sébert, S. P. et al. Maternal nutrient restriction between early-to-mid gestation and its impact upon appetite regulation following juvenile obesity. Endocrinology 150, 634–641 (2009).
Park, J. H., Stoffers, D. A., Nicholls, R. D. & Simmons, R. A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Invest. 118, 2316–2324 (2008).
Blondeau, B., Avril, I., Duchene, B. & Bréant, B. Endocrine pancreas development is altered in foetuses from rats previously showing intra-uterine growth retardation in response to malnutrition. Diabetologia 45, 394–401 (2002).
Sharkey, D. et al. Maternal nutrient restriction during pregnancy differentially alters the unfolded protein response in adipose and renal tissue of obese juvenile offspring. FASEB J. 23, 1314–1324 (2009).
Tarry-Adkins, J. L. et al. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 23, 1521–1528 (2009).
Mühlhäusler, B. S. et al. Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. J. Physiol. 565, 185–195 (2005).
Chan, L. L. et al. Effect of maternal nutrient restriction from early to midgestation on cardiac function and metabolism after adolescent-onset obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1455–R1463 (2009).
Zawia, N. H., Lahiri, D. K. & Cardozo-Pelaez, F. Epigenetics, oxidative stress, and Alzheimer disease. Free Radic. Biol. Med. 46, 1241–1249 (2009).
Percy, C. J., Brown, L., Power, D. A., Johnson, D. W. & Gobe, G. C. Obesity and hypertension have differing oxidant handling molecular pathways in age-related chronic kidney disease. Mech. Ageing Dev. 130, 129–138 (2009).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009).
Zhu, M. J. et al. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J. Physiol. 586, 2651–2664 (2008).
Yamauchi, T. et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 13, 332–339 (2007).
Boden, G. et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 (2008).
Yajnik, C. S. et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 51, 29–38 (2008).
Acknowledgements
The authors acknowledge the support of the European Union Sixth Framework Program for Research and Technical Development of the European Community—The Early Nutrition Programming Project (FOOD-CT-2005-007,036)—in their research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Symonds, M., Sebert, S., Hyatt, M. et al. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol 5, 604–610 (2009). https://doi.org/10.1038/nrendo.2009.195
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.195
This article is cited by
-
Early starvation in European seabass (Dicentrarchus labrax) larvae has no drastic effect on hepatic intermediary metabolism in juveniles
Fish Physiology and Biochemistry (2024)
-
Associations of maternal non-nutritive sweetener intake during pregnancy with offspring body mass index and body fat from birth to adolescence
International Journal of Obesity (2022)
-
Nutritional programming by maternal diet alters offspring lipid metabolism in a marine teleost
Fish Physiology and Biochemistry (2022)
-
Maternal nutrition and its intergenerational links to non-communicable disease metabolic risk factors: a systematic review and narrative synthesis
Journal of Health, Population and Nutrition (2021)
-
Dietary curcumin supplementation promotes browning and energy expenditure in postnatal overfed rats
Nutrition & Metabolism (2021)