Abstract
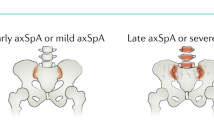
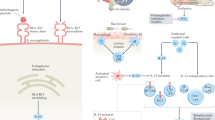
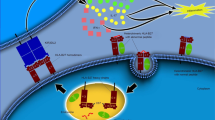
The term axial spondyloarthritis covers both non-radiographic disease and radiographic disease (also known as ankylosing spondylitis). Some studies have been performed to investigate the prevalence of axial spondyloarthritis, although most are limited to patients with radiographic disease. A strong genetic association has been shown between axial spondyloarthritis and human leukocyte antigen-B27 (HLA-B27), but the pathogenetic role of HLA-B27 has not yet been clarified. Tumour necrosis factor (TNF), IL-17, IL-23 and downstream pathways also seem to be important — based on the good results of therapies directed against these molecules — but their exact role in the inflammatory process is also not yet clear. Elucidating the interaction between osteoproliferation and inflammation will be crucial for the prevention of long-term structural damage of the bone. The development of new criteria for classification, diagnosis and screening of patients with axial spondyloarthritis will enable earlier intervention for this chronic inflammatory disease. MRI has become an important tool for the early detection of axial spondyloarthritis. NSAIDs and TNF blockers are effective therapies, including in the early non-radiographic stage. Therapeutic blockade of IL-17 or IL-23 seems to be a promising new treatment option. Tools for measuring quality of life in axial spondyloarthritis have become relevant to assess the impact that the disease has on patients. These diagnostic and therapeutic advances will continue to change the management of axial spondyloarthritis, and new insights into the disease pathogenesis will hopefully accelerate this process. For an illustrated summary of this Primer, visit: http://go.nature.com/51b1af
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Moll, J. M., Haslock, I., Macrae, I. F. & Wright, V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter's disease, the intestinal arthropathies, and Behcet's syndrome. Medicine 53, 343–364 (1974).
Dougados, M. et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 34, 1218–1227 (1991).
Amor, B., Dougados, M. & Mijiyawa, M. [Criteria of the classification of spondylarthropathies]. Rev. Rhum. Mal. Osteoartic. 57, 85–89 (in French) (1990).
Rudwaleit, M. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann. Rheum. Dis. 68, 777–783 (2009).
Rudwaleit, M. et al. The Assessment of SpondyloArthritis international Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 70, 25–31 (2011).
Sieper, J. & van der Heijde, D. Nonradiographic axial spondyloarthritis: new definition of an old disease? Arthritis Rheum. 65, 543–551 (2013).
Saraux, A. et al. Prevalence of spondyloarthropathies in France: 2001. Ann. Rheum. Dis. 64, 1431–1435 (2005).
Guillemin, F. et al. Prevalence of rheumatoid arthritis in France: 2001. Ann. Rheum. Dis. 64, 1427–1430 (2005).
Robinson, P. C. & Brown, M. A. Genetics of ankylosing spondylitis. Mol. Immunol. 57, 2–11 (2014).
Dean, L. E. et al. Global prevalence of ankylosing spondylitis. Rheumatology 53, 650–657 (2014).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
Stolwijk, C., Boonen, A., van Tubergen, A. & Reveille, J. D. Epidemiology of spondyloarthritis. Rheum. Dis. Clin. North Am. 38, 441–476 (2012).
Reveille, J. D., Witter, J. P. & Weisman, M. H. Prevalence of axial spondylarthritis in the United States: estimates from a cross-sectional survey. Arthritis Care Res. 64, 905–910 (2012). This epidemiological study found a high prevalence of axial spondyloarthritis in the United States.
Rudwaleit, M. et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 60, 717–727 (2009).
Dougados, M. et al. The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine 78, 598–603 (2011).
Chung, H. Y., Machado, P., van der Heijde, D., D'Agostino, M.-A. & Dougados, M. HLA-B27 positive patients differ from HLA-B27 negative patients in clinical presentation and imaging: results from the DESIR cohort of patients with recent onset axial spondyloarthritis. Ann. Rheum. Dis. 70, 1930–1936 (2011).
Ciurea, A. et al. Age at symptom onset in ankylosing spondylitis: is there a gender difference? Ann. Rheum. Dis. 73, 1908–1910 (2014).
Ramiro, S. et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann. Rheum. Dis. 74, 52–59 (2015). This long-term follow-up study showed a correlation between persistent disease activity and radiographic progression for the first time.
Chung, H. Y., Machado, P., van der Heijde, D., D'Agostino, M.-A. & Dougados, M. Smokers in early axial spondyloarthritis have earlier disease onset, more disease activity, inflammation and damage, and poorer function and health-related quality of life: results from the DESIR cohort. Ann. Rheum. Dis. 71, 809–816 (2012).
Poddubnyy, D. et al. Cigarette smoking has a dose-dependent impact on progression of structural damage in the spine in patients with axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort (GESPIC). Ann. Rheum. Dis. 72, 1430–1432 (2013).
Videm, V., Cortes, A., Thomas, R. & Brown, M. A. Current smoking is associated with incident ankylosing spondylitis — the HUNT population-based Norwegian health study. J. Rheumatol. 41, 2041–2048 (2014). This study suggests that smoking might not only be associated with a more-severe disease and/or a more-progressive disease but also with a higher incidence of the disease.
Robinson, P. C. & Brown, M. A. The genetics of ankylosing spondylitis and axial spondyloarthritis. Rheum. Dis. Clin. North Am. 38, 539–553 (2012).
Mijiyawa, M., Oniankitan, O. & Khan, M. A. Spondyloarthropathies in sub-Saharan Africa. Curr. Opin. Rheumatol. 12, 281–286 (2000).
Asquith, M., Elewaut, D., Lin, P. & Rosenbaum, J. T. The role of the gut and microbes in the pathogenesis of spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 28, 687–702 (2014).
Claudepierre, P. et al. Predictive factors of severity of spondyloarthropathy in North Africa. Br. J. Rheumatol. 34, 1139–1145 (1995).
McGonagle, D. & McDermott, M. F. A proposed classification of the immunological diseases. PLoS Med. 3, e297 (2006).
International Genetics of Ankylosing Spondylitis Consortium (IGAS). Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730–738 (2013). This paper provides a state-of-the-art overview of the genetic risk factors for ankylosing spondylitis, thereby identifying cellular and molecular pathways that might be involved in the pathogenesis of the disease.
Song, I.-H. et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum. 62, 1290–1297 (2010).
Song, I.-H. et al. Treatment of active ankylosing spondylitis with abatacept: an open-label, 24-week pilot study. Ann. Rheum. Dis. 70, 1108–1110 (2011).
Baraliakos, X., Baerlecken, N., Witte, T., Heldmann, F. & Braun, J. High prevalence of anti-CD74 antibodies specific for the HLA class II-associated invariant chain peptide (CLIP) in patients with axial spondyloarthritis. Ann. Rheum. Dis. 73, 1079–1082 (2014).
Baerlecken, N. T. et al. Autoantibodies against CD74 in spondyloarthritis. Ann. Rheum. Dis. 73, 1211–1214 (2014).
Park, H., Bourla, A. B., Kastner, D. L., Colbert, R. A. & Siegel, R. M. Lighting the fires within: the cell biology of autoinflammatory diseases. Nat. Rev. Immunol. 12, 570–580 (2012). This paper reviews how HLA-B27 could contribute to the pathogenesis of axial spondyloarthritis in an antigen-independent manner.
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Ambarus, C., Yeremenko, N., Tak, P. P. & Baeten, D. Pathogenesis of spondyloarthritis: autoimmune or autoinflammatory? Curr. Opin. Rheumatol. 24, 351–358 (2012).
Dougados, M. & Baeten, D. Spondyloarthritis. Lancet 377, 2127–2137 (2011).
Jacques, P., Elewaut, D. & Mielants, H. Interactions between gut inflammation and arthritis/spondylitis. Curr. Opin. Rheumatol. 22, 368–374 (2010).
Jacques, P. et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann. Rheum. Dis. 73, 437–445 (2014).
McGonagle, D., Gibbon, W. & Emery, P. Classification of inflammatory arthritis by enthesitis. Lancet 352, 1137–1140 (1998).
McGonagle, D. et al. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondylarthropathy. Arthritis Rheum. 41, 694–700 (1998).
Paramarta, J. E. et al. Peripheral joint inflammation in early onset spondyloarthritis is not specifically related to enthesitis. Ann. Rheum. Dis. 73, 735–740 (2014).
Lories, R. J., Matthys, P., de Vlam, K., Derese, I. & Luyten, F. P. Ankylosing enthesitis, dactylitis, and onychoperiostitis in male DBA/1 mice: a model of psoriatic arthritis. Ann. Rheum. Dis. 63, 595–598 (2004).
McGonagle, D. et al. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann. Rheum. Dis. 61, 534–537 (2002).
Baeten, D. et al. Association of CD163+ macrophages and local production of soluble CD163 with decreased lymphocyte activation in spondylarthropathy synovitis. Arthritis Rheum. 50, 1611–1623 (2004).
Baeten, D. et al. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res. Ther. 7, R359–R369 (2005).
van Duivenvoorde, L. M. et al. Innate immune stimulation triggers early-onset spondyloarthritis in HLA-B27/human Beta2 microglobulin transgenic rats. Arthritis Rheum. Abstr. 63, 990 (2011).
Ruutu, M. et al. β-glucan triggers spondylarthritis and Crohn's disease-like ileitis in SKG mice. Arthritis Rheum. 64, 2211–2222 (2012).
Khan, M. A. HLA-B27 and its subtypes in world populations. Curr. Opin. Rheumatol. 7, 263–269 (1995).
Khan, M. A. Polymorphism of HLA-B27: 105 subtypes currently known. Curr. Rheumatol. Rep. 15, 362 (2013).
Hammer, R. E., Maika, S. D., Richardson, J. A., Tang, J. P. & Taurog, J. D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell 63, 1099–1112 (1990). This seminal paper proves the functional involvement of HLA-B27 in the pathogenesis of axial spondyloarthritis and describes the key animal model for the study of disease pathophysiology.
Hermann, E., Yu, D. T., Meyer zum Bü schenfelde, K. H. & Fleischer, B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet 342, 646–650 (1993).
Atagunduz, P. et al. HLA-B27-restricted CD8+ T cell response to cartilage-derived self peptides in ankylosing spondylitis. Arthritis Rheum. 52, 892–901 (2005).
Evans, D. M. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat. Genet. 43, 761–767 (2011).
Taurog, J. D. et al. Spondylarthritis in HLA-B27/human β2-microglobulin-transgenic rats is not prevented by lack of CD8. Arthritis Rheum. 60, 1977–1984 (2009).
Mear, J. P. et al. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol. 163, 6665–6670 (1999).
DeLay, M. L. et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 60, 2633–2643 (2009).
Goodall, J. C. et al. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl Acad. Sci. USA 107, 17698–17703 (2010).
Turner, M. J. et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J. Immunol. 175, 2438–2448 (2005).
Neerinckx, B., Carter, S. & Lories, R. J. No evidence for a critical role of the unfolded protein response in synovium and blood of patients with ankylosing spondylitis. Ann. Rheum. Dis. 73, 629–630 (2014).
Kollnberger, S. et al. Cell-surface expression and immune receptor recognition of HLA-B27 homodimers. Arthritis Rheum. 46, 2972–2982 (2002).
Chan, A. T., Kollnberger, S. D., Wedderburn, L. R. & Bowness, P. Expansion and enhanced survival of natural killer cells expressing the killer immunoglobulin-like receptor KIR3DL2 in spondylarthritis. Arthritis Rheum. 52, 3586–3595 (2005).
Bowness, P. et al. Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers are increased in ankylosing spondylitis. J. Immunol. 186, 2672–2680 (2011).
Wu, H.-J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Lin, P. et al. HLA-B27 and human β2-microglobulin affect the gut microbiota of transgenic rats. PLoS ONE 9, e105684 (2014).
Sahlberg, A. S., Granfors, K. & Penttinen, M. A. HLA-B27 and host–pathogen interaction. Adv. Exp. Med. Biol. 649, 235–244 (2009).
Zeidler, H. & Hudson, A. P. New insights into Chlamydia and arthritis. Promise of a cure?. Ann. Rheum. Dis. 73, 637–644 (2014).
Tran, T. M. et al. Additional human β2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 54, 1317–1327 (2006).
Coffre, M. et al. Combinatorial control of Th17 and Th1 cell functions by genetic variations in genes associated with the interleukin-23 signaling pathway in spondyloarthritis. Arthritis Rheum. 65, 1510–1521 (2013).
Jandus, C. et al. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 58, 2307–2317 (2008).
Kenna, T. J. et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 64, 1420–1429 (2012).
Appel, H. et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Noordenbos, T. et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 64, 99–109 (2012).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−,CD8−, entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Adamopoulos, I. E. et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 187, 951–959 (2011).
Benham, H. et al. Interleukin-23 mediates the intestinal response to microbial β-1,3-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol. 66, 1755–1767 (2014).
Braun, J. et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 38, 499–505 (1995).
Kontoyiannis, D., Pasparakis, M., Pizarro, T. T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Armaka, M. et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med. 205, 331–337 (2008).
Wanders, A. et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 52, 1756–1765 (2005).
Vandooren, B. et al. Mediators of structural remodeling in peripheral spondylarthritis. Arthritis Rheum. 60, 3534–3545 (2009).
Herman, S., Krönke, G. & Schett, G. Molecular mechanisms of inflammatory bone damage: emerging targets for therapy. Trends Mol. Med. 14, 245–253 (2008).
François, R. J., Gardner, D. L., Degrave, E. J. & Bywaters, E. G. Histopathologic evidence that sacroiliitis in ankylosing spondylitis is not merely enthesitis. Arthritis Rheum. 43, 2011–2024 (2000).
van Duivenvoorde, L. M. et al. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA-B27/human β2-microglobulin-transgenic rat model of spondylarthritis. Arthritis Rheum. 64, 3210–3219 (2012).
Diarra, D. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163 (2007).
Daoussis, D. et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 62, 150–158 (2010).
Heiland, G. R. et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann. Rheum. Dis. 71, 572–574 (2012).
Appel, H. et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 60, 3257–3262 (2009).
Lories, R. J., Derese, I. & Luyten, F. P. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J. Clin. Invest. 115, 1571–1579 (2005).
van der Heijde, D. et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 58, 3063–3070 (2008).
Baraliakos, X., Haibel, H., Listing, J., Sieper, J. & Braun, J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann. Rheum. Dis. 73, 710–715 (2014).
Haroon, N. et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 65, 2645–2654 (2013).
Sieper, J., Appel, H., Braun, J. & Rudwaleit, M. Critical appraisal of assessment of structural damage in ankylosing spondylitis: implications for treatment outcomes. Arthritis Rheum. 58, 649–656 (2008).
Lories, R. J., Derese, I. & Luyten, F. P. Inhibition of osteoclasts does not prevent joint ankylosis in a mouse model of spondyloarthritis. Rheumatology 47, 605–608 (2008).
Lories, R. J., Luyten, F. P. & de Vlam, K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res. Ther. 11, 221 (2009).
Yeremenko, N. et al. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum. 65, 174–185 (2013).
van Duivenvoorde, L. M., van Tok, M. N. & Baeten, D. L. Membrane-bound TNF drives axial and peripheral inflammation and pathologic new bone formation. Arthritis Rheum. Abstr. 65 (Suppl. 10), 536 (2009).
Sieper, J., Braun, J., Rudwaleit, M., Boonen, A. & Zink, A. Ankylosing spondylitis: an overview. Ann. Rheum. Dis. 61, iii8–iii18 (2002).
Rudwaleit, M., Khan, M. A. & Sieper, J. The challenge of diagnosis and classification in early ankylosing spondylitis: do we need new criteria? Arthritis Rheum. 52, 1000–1008 (2005).
Braun, J., Baraliakos, X., Kiltz, U., Heldmann, F. & Sieper, J. Classification and diagnosis of axial spondyloarthritis — what is the clinically relevant difference? J. Rheumatol. 42, 31–38 (2015). This paper explains the clinically relevant differences between classification and diagnosis of patients with axial spondyloarthritis in a practical way.
van den Berg, R. et al. ASAS modification of the Berlin algorithm for diagnosing axial spondyloarthritis: results from the SPondyloArthritis Caught Early (SPACE)-cohort and from the Assessment of SpondyloArthritis international Society (ASAS)-cohort. Ann. Rheum. Dis. 72, 1646–1653 (2013). This is a proposal for a diagnostic algorithm for axial spondyloarthritis that can be applied in daily clinical practice.
Rudwaleit, M. et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 68, 1520–1527 (2009).
Sieper, J. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann. Rheum. Dis. 68, ii1–ii44 (2009).
Weber, U. et al. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res. 62, 1763–1771 (2010).
van den Berg, R. et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheumatol. 66, 2403–2411 (2014).
Mandl, P. et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 74, 1327–1339 (2015).
Sieper, J. & Rudwaleit, M. Early referral recommendations for ankylosing spondylitis (including pre-radiographic and radiographic forms) in primary care. Ann. Rheum. Dis. 64, 659–663 (2005).
Hermann, J., Giessauf, H., Schaffler, G., Ofner, P. & Graninger, W. Early spondyloarthritis: usefulness of clinical screening. Rheumatology 48, 812–816 (2009).
Sieper, J. et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: the Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann. Rheum. Dis. 72, 1621–1627 (2013).
Braun, A. et al. Optimizing the identification of patients with axial spondyloarthritis in primary care—the case for a two-step strategy combining the most relevant clinical items with HLA B27. Rheumatology 52, 1418–1424 (2013).
Rudwaleit, M. & Sieper, J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat. Rev. Rheumatol. 8, 262–268 (2012).
Deodhar, A. et al. Prevalence of axial spondyloarthritis among undiagnosed chronic back pain patients in the United States. Ann. Rheum. Dis. 73, 198–199 (2014).
Poddubnyy, D., Rudwaleit, M., Haibel, H. & Landewé, R. xs Assessment of Spondyloarthritis international Society endorsed recommendations for early referral of patients suspected for axial spondyloarthritis. Arthritis Rheumatol. 66, 1133–1134 (2014).
Sieper, J. et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann. Rheum. Dis. 68, 784–788 (2009).
Baraliakos, X. et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann. Rheum. Dis. 66, 910–915 (2007).
Heuft-Dorenbosch, L. et al. Combining information obtained from magnetic resonance imaging and conventional radiographs to detect sacroiliitis in patients with recent onset inflammatory back pain. Ann. Rheum. Dis. 65, 804–808 (2006).
Poddubnyy, D. et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann. Rheum. Dis. 70, 1369–1374 (2011).
Braun, J. et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 359, 1187–1193 (2002).
Gran, J. T. & Skomsvoll, J. F. The outcome of ankylosing spondylitis: a study of 100 patients. Br. J. Rheumatol. 36, 766–771 (1997).
van der Heijde, D. et al. Evaluation of the efficacy of etoricoxib in ankylosing spondylitis: results of a fifty-two-week, randomized, controlled study. Arthritis Rheum. 52, 1205–1215 (2005).
Amor, B. et al. Are classification criteria for spondylarthropathy useful as diagnostic criteria? Rev. Rhum. Engl. Ed. 62, 10–15 (1995).
Amor, B. [Response to treatment as an aid to diagnosis]. Rev. Rhum. Mal. Osteoartic. 59, 3S–6S (in French) (1992).
Dougados, M. et al. ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann. Rheum. Dis. 70, 249–251 (2011).
Inman, R. D. et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, Phase III trial. Arthritis Rheum. 58, 3402–3412 (2008).
Sieper, J. et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: results from the double-blind, placebo-controlled INFAST study, part 1. Ann. Rheum. Dis. 73, 101–107 (2014).
Smolen, J. S., van der Heijde, D., Machold, K. P., Aletaha, D. & Landewé, R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 73, 3–5 (2014).
van der Horst-Bruinsma, I. E., Clegg, D. O. & Dijkmans, B. A. Treatment of ankylosing spondylitis with disease modifying antirheumatic drugs. Clin. Exp. Rheumatol. 20, S67–S70 (2002).
Nissilä, M. et al. Sulfasalazine in the treatment of ankylosing spondylitis. A twenty-six-week, placebo-controlled clinical trial. Arthritis Rheum. 31, 1111–1116 (1988).
Braun, J. et al. Efficacy of sulfasalazine in patients with inflammatory back pain due to undifferentiated spondyloarthritis and early ankylosing spondylitis: a multicentre randomised controlled trial. Ann. Rheum. Dis. 65, 1147–1153 (2006).
Breban, M. et al. Maintenance of infliximab treatment in ankylosing spondylitis: results of a one-year randomized controlled trial comparing systematic versus on-demand treatment. Arthritis Rheum. 58, 88–97 (2008).
Haibel, H. et al. Efficacy of oral prednisolone in active ankylosing spondylitis: results of a double-blind, randomised, placebo-controlled short-term trial. Ann. Rheum. Dis. 73, 243–246 (2014).
van der Heijde, D. et al. 2010 update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann. Rheum. Dis. 70, 905–908 (2011).
Gorman, J. D., Sack, K. E. & Davis, J. C. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor α. N. Engl. J. Med. 346, 1349–1356 (2002).
Sieper, J. et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann. Rheum. Dis. 72, 815–822 (2013).
Landewé, R. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann. Rheum. Dis. 73, 39–47 (2014).
Braun, J. et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum. 48, 1126–1136 (2003).
Braun, J. et al. Persistent clinical response to the anti-TNF-α antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology 44, 670–676 (2005).
van der Heijde, D. et al. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann. Rheum. Dis. 68, 922–929 (2009).
Rudwaleit, M., Listing, J., Brandt, J., Braun, J. & Sieper, J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor α blockers in ankylosing spondylitis. Ann. Rheum. Dis. 63, 665–670 (2004).
Vastesaeger, N. et al. Predicting the outcome of ankylosing spondylitis therapy. Ann. Rheum. Dis. 70, 973–981 (2011).
Carmona, L. et al. Safety and retention rate of off-label uses of TNF antagonists in rheumatic conditions: data from the Spanish registry BIOBADASER 2. 0. Rheumatology 50, 85–92 (2011).
Baraliakos, X. et al. Clinical response to discontinuation of anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res. Ther. 7, R439–R444 (2005).
Haibel, H. et al. Long-term efficacy of adalimumab after drug withdrawal and retreatment in patients with active non-radiographically evident axial spondyloarthritis who experience a flare. Arthritis Rheum. 65, 2211–2213 (2013).
Arends, S. et al. Patient-tailored dose reduction of TNF-α blocking agents in ankylosing spondylitis patients with stable low disease activity in daily clinical practice. Clin. Exp. Rheumatol. 33,174–180 (2015).
Braun, J. et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann. Rheum. Dis. 70, 896–904 (2011).
Dougados, M. et al. Nonsteroidal antiinflammatory drug intake according to the Assessment of SpondyloArthritis international Society score in clinical trials evaluating tumor necrosis factor blockers: example of etanercept in advanced ankylosing spondylitis. Arthritis Care Res. 64, 290–294 (2012).
Haibel, H., Rudwaleit, M., Listing, J. & Sieper, J. Open label trial of anakinra in active ankylosing spondylitis over 24 weeks. Ann. Rheum. Dis. 64, 296–298 (2005).
Sieper, J. et al. Sarilumab for the treatment of ankylosing spondylitis: results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann. Rheum. Dis. 74, 1051–1057 (2015).
Sieper, J., Porter-Brown, B., Thompson, L., Harari, O. & Dougados, M. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann. Rheum. Dis. 73, 95–100 (2014).
Song, I.-H. et al. Major clinical response of rituximab in active TNF-blocker-naive patients with ankylosing spondylitis but not in TNF-blocker-failure patients — an open label clinical trial. Arthritis Rheum. Abstr. 60 (Suppl.10), 1769 (2009).
Baeten, D. et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713 (2013). This trial provides the first evidence that strategies targeting IL-17 work in ankylosing spondylitis.
Poddubnyy, D., Hermann, K.-G., Callhoff, J., Listing, J. & Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 73, 817–823 (2014). This trial provides the first evidence that strategies targeting IL-23 work in ankylosing spondylitis.
Baeten, D. L. et al. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a 52-week Phase 3 randomized placebo-controlled trial with intravenous loading and subcutaneous maintenance dosing. Am. Coll. Rheumatol. Abstr.[online] (2014).
Sieper, J. et al. Secukinumab, a monoclonal antibody to interleukin-17A, significantly improves signs and symptoms of active ankylosing spondylitis: results of a Phase 3, randomized, placebo-controlled trial with subcutaneous loading and maintenance dosing. Am. Coll. Rheumatol. Abstr.[online] (2014).
Sandborn, W. J. et al. A randomized trial of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology 135, 1130–1141 (2008).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Dagfinrud, H., Kvien, T. K. & Hagen, K. B. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst. Rev. 23, CD002822 (2008).
Lukas, C. et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann. Rheum. Dis. 68, 18–24 (2009).
Smolen, J. S. et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann. Rheum. Dis. 73, 6–16 (2014).
Gill, T. M. & Feinstein, A. R. A critical appraisal of the quality of quality-of-life measurements. JAMA 272, 619–626 (1994).
Guyatt, G. H., Feeny, D. H. & Patrick, D. L. Measuring health-related quality of life. Ann. Intern. Med. 118, 622–629 (1993).
Chorus, A. M., Miedema, H. S., Boonen, A. & Van Der Linden, S. Quality of life and work in patients with rheumatoid arthritis and ankylosing spondylitis of working age. Ann. Rheum. Dis. 62, 1178–1184 (2003).
Kotsis, K., Voulgari, P. V., Drosos, A. A., Carvalho, A. F. & Hyphantis, T. Health-related quality of life in patients with ankylosing spondylitis: a comprehensive review. Expert Rev. Pharmacoecon. Outcomes Res. 14, 857–872 (2014).
Salaffi, F., Carotti, M., Gasparini, S., Intorcia, M. & Grassi, W. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual. Life Outcomes 7, 25 (2009).
Kiltz, U. & van der Heijde, D. Health-related quality of life in patients with rheumatoid arthritis and in patients with ankylosing spondylitis. Clin. Exp. Rheumatol. 27, S108–S111 (2009).
Boonen, A. et al. Rapid and sustained improvement in health-related quality of life and utility for 72 weeks in patients with ankylosing spondylitis receiving etanercept. J. Rheumatol. 35, 662–667 (2008).
van der Heijde, D. et al. The effect of golimumab therapy on disease activity and health-related quality of life in patients with ankylosing spondylitis: 2-year results of the GO-RAISE trial. J. Rheumatol. 41, 1095–1103 (2014).
Martindale, J. et al. Disease and psychological status in ankylosing spondylitis. Rheumatology 45, 1288–1293 (2006).
Ward, M. M., Reveille, J. D., Learch, T. J., Davis, J. C. & Weisman, M. H. Impact of ankylosing spondylitis on work and family life: comparisons with the US population. Arthritis Rheum. 59, 497–503 (2008).
Ariza-Ariza, R., Hernández-Cruz, B. & Navarro-Sarabia, F. Physical function and health-related quality of life of Spanish patients with ankylosing spondylitis. Arthritis Rheum. 49, 483–487 (2003).
Ware, J. E. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483 (1992).
Doward, L. C. et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann. Rheum. Dis. 62, 20–26 (2003).
Khanna, D. & Tsevat, J. Health-related quality of life—an introduction. Am. J. Manag. Care 13, S218–S223 (2007).
van Echteld, I. et al. Identification of the most common problems by patients with ankylosing spondylitis using the international classification of functioning, disability and health. J. Rheumatol. 33, 2475–2483 (2006).
Boonen, A. et al. ASAS/WHO ICF Core Sets for ankylosing spondylitis (AS): how to classify the impact of AS on functioning and health. Ann. Rheum. Dis. 69, 102–107 (2010).
Kiltz, U. et al. Development of a health index in patients with ankylosing spondylitis (ASAS HI): final result of a global initiative based on the ICF guided by ASAS. Ann. Rheum. Dis. 74, 830–835 (2015). The development of a health index in axial spondyloarthritis is a major step forward in the assessment of the burden of disease in these patients.
Gossec, L. et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann. Rheum. Dis. 73, 1012–1019 (2014).
Brandt, J. et al. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor α monoclonal antibody infliximab. Arthritis Rheum. 43, 1346–1352 (2000).
Helmick, C. G. et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 58, 15–25 (2008).
Reveille, J. D., Hirsch, R., Dillon, C. F., Carroll, M. D. & Weisman, M. H. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum. 64, 1407–1411 (2012).
van der Linden, S. M., Valkenburg, H. A., de Jongh, B. M. & Cats, A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals. A comparison of relatives of spondylitis patients with the general population. Arthritis Rheum. 27, 241–249 (1984).
Akkoc, N. & Khan, M. A. Overestimation of the prevalence of ankylosing spondylitis in the Berlin study: comment on the article by Braun et al. Arthritis Rheum. 52, 4048–4049; author reply 4049–4050 (2005).
Gran, J. T., Husby, G. & Hordvik, M. Prevalence of ankylosing spondylitis in males and females in a young middle-aged population of Tromsø, northern Norway. Ann. Rheum. Dis. 44, 359–367 (1985).
Gofton, J. P., Robinson, H. S. & Trueman, G. E. Ankylosing spondylitis in a Canadian Indian population. Ann. Rheum. Dis. 25, 525–527 (1966).
Acknowledgements
The authors thank U. Kiltz and X. Baraliakos for their support in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Introduction (J.S.); Epidemiology (M.D.); Mechanisms/pathophysiology (D.B.); Diagnosis, screening and prevention (J.S.); Management (J.B.); Quality of life (J.B.); Outlook (J.S.); overview of Primer (J.S.).
Corresponding author
Ethics declarations
Competing interests
J.S. has received honoraria for being a member of speaker's bureau and/or consulting fees from the following companies: AbbVie Boehringer Ingelheim Janssen Lilly Merck Novartis Pfizer and UCB; and research grants from the following companies: AbbVie Janssen Merck and Pfizer. J.B. discloses no financial conflicts. M.D. has received honouraria for being a member of speaker's bureau and/or consulting fees from the following companies: AbbVie Boehringer Ingelheim Janssen Lilly Merck Novartis Pfizer and UCB; and his department has received research grants from the following companies: AbbVie Janssen Lilly Merck Pfizer Novartis and UCB. D.B. has received honouraria for being a member of speakers bureau and/or consulting fees from the following companies: AbbVie Pfizer MSD UCB Novartis Lilly Boehringer Ingelheim Roche Bristol–Myers Squibb Janssen Glenmark Zymetech and FivePrime.
Rights and permissions
About this article
Cite this article
Sieper, J., Braun, J., Dougados, M. et al. Axial spondyloarthritis. Nat Rev Dis Primers 1, 15013 (2015). https://doi.org/10.1038/nrdp.2015.13
Published:
DOI: https://doi.org/10.1038/nrdp.2015.13
This article is cited by
-
Quantitative prediction of radiographic progression in patients with axial spondyloarthritis using neural network model in a real-world setting
Arthritis Research & Therapy (2023)
-
Proposal of simplified CT syndesmophyte score (sCTSS) and comparison with CTSS in patients with ankylosing spondylitis
Scientific Reports (2023)
-
A Cross-Indication Budget Impact Model of Secukinumab for the Treatment of Psoriasis, Psoriatic Arthritis, Ankylosing Spondylitis and Non-radiographic Axial Spondyloarthritis in Italy
PharmacoEconomics - Open (2023)
-
Imrecoxib and celecoxib affect sacroiliac joint inflammation in axSpA by regulating bone metabolism and angiogenesis
Clinical Rheumatology (2023)
-
Neuropathic pain in axial spondyloarthropathy is underdiagnosed and a confounding factor in biologic drug–switching decision: a cross-sectional study
Clinical Rheumatology (2023)