Abstract
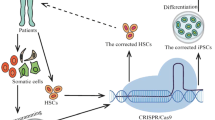
Genome editing via homologous recombination (HR) (gene targeting) in human hematopoietic stem cells (HSCs) has the power to reveal gene–function relationships and potentially transform curative hematological gene and cell therapies. However, there are no comprehensive and reproducible protocols for targeting HSCs for HR. Herein, we provide a detailed protocol for the production, enrichment, and in vitro and in vivo analyses of HR-targeted HSCs by combining CRISPR/Cas9 technology with the use of rAAV6 and flow cytometry. Using this protocol, researchers can introduce single-nucleotide changes into the genome or longer gene cassettes with the precision of genome editing. Along with our troubleshooting and optimization guidelines, researchers can use this protocol to streamline HSC genome editing at any locus of interest. The in vitro HSC-targeting protocol and analyses can be completed in 3 weeks, and the long-term in vivo HSC engraftment analyses in immunodeficient mice can be achieved in 16 weeks. This protocol enables manipulation of genes for investigation of gene functions during hematopoiesis, as well as for the correction of genetic mutations in HSC transplantation–based therapies for diseases such as sickle cell disease, β-thalassemia, and primary immunodeficiencies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Porteus, M. Genome editing: a new approach to human therapeutics. Annu. Rev. Pharmacol. Toxicol. 56, 163–190 (2016).
Porteus, M.H. Towards a new era in medicine: therapeutic genome editing. Genome Biol. 16, 286 (2015).
Naldini, L. Ex vivo gene transfer and correction for cell-based therapies. Nat. Rev. Genet. 12, 301–315 (2011).
Cox, D.B.T., Platt, R.J. & Zhang, F. Therapeutic genome editing: prospects and challenges. Nat. Med. 21, 121–131 (2015).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Hendel, A. et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 33, 985–989 (2015).
Osborn, M.J. et al. Evaluation of TCR gene editing achieved by TALENs, CRISPR/Cas9, and megaTAL nucleases. Mol. Ther. 24, 570–581 (2016).
Schumann, K. et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl. Acad. Sci. USA 112, 10437–10442 (2015).
DeWitt, M.A. et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci. Transl. Med. 8, 360ra134 (2016).
Gundry, M.C. et al. Highly efficient genome editing of murine and human hematopoietic progenitor cells by CRISPR/Cas9. Cell Rep. 17, 1453–1461 (2016).
Porteus, M.H. & Baltimore, D. Chimeric nucleases stimulate gene targeting in human cells. Science 300, 763 (2003).
Rouet, P., Smih, F. & Jasin, M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl. Acad. Sci. USA 91, 6064–6068 (1994).
Kass, E.M. & Jasin, M. Collaboration and competition between DNA double-strand break repair pathways. FEBS Lett. 584, 3703–3708 (2010).
Dever, D.P. et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature 539, 384–389 (2016).
Hendel, A. et al. Quantifying genome-editing outcomes at endogenous loci with SMRT sequencing. Cell Rep. 7, 293–305 (2014).
Barzel, A. et al. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature 517, 360–364 (2015).
Miller, D.G., Petek, L.M. & Russell, D.W. Human gene targeting by adeno-associated virus vectors is enhanced by DNA double-strand breaks. Mol. Cell. Biol. 23, 3550–3557 (2003).
Russell, D.W. & Hirata, R.K. Human gene targeting by viral vectors. Nat. Genet. 18, 325–330 (1998).
Song, L. et al. High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS One 8, e58757 (2013).
Wang, J. et al. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 44, e30 (2016).
Sather, B.D. et al. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci. Transl. Med. 7, 307ra156 (2015).
De Ravin, S.S. et al. Targeted gene addition in human CD34+ hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat. Biotechnol. 34, 424–429 (2016).
De Ravin, S.S. et al. CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci. Transl. Med. 9, aah3480 (2017).
Baum, C.M., Weissman, I.L., Tsukamoto, A.S., Buckle, A.M. & Peault, B. Isolation of a candidate human hematopoietic stem-cell population. Proc. Natl. Acad. Sci. USA 89, 2804–2808 (1992).
Doulatov, S., Notta, F., Laurenti, E. & Dick, J.E. Hematopoiesis: a human perspective. Cell Stem Cell 10, 120–136 (2012).
Majeti, R., Park, C.Y. & Weissman, I.L. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1, 635–645 (2007).
Notta, F. et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333, 218–221 (2011).
Shultz, L.D., Ishikawa, F. & Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Genovese, P. et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510, 235–240 (2014).
Hoban, M.D. et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood 125, 2597–2604 (2015).
Bak, R.O. & Porteus, M.H. CRISPR-mediated integration of large gene cassettes using AAV donor vectors. Cell Rep. 20, 750–756 (2017).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Ran, F.A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Kleinstiver, B.P. et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 523, 481–485 (2015).
Elliott, B., Richardson, C., Winderbaum, J., Nickoloff, J.A. & Jasin, M. Gene conversion tracts from double-strand break repair in mammalian cells. Mol. Cell. Biol. 18, 93–101 (1998).
Rago, C., Vogelstein, B. & Bunz, F. Genetic knockouts and knockins in human somatic cells. Nat. Protoc. 2, 2734–2746 (2007).
Khan, I.F., Hirata, R.K. & Russell, D.W. AAV-mediated gene targeting methods for human cells. Nat. Protoc. 6, 482–501 (2011).
Hirata, R.K. & Russell, D.W. Design and packaging of adeno-associated virus gene targeting vectors. J. Virol. 74, 4612–4620 (2000).
Hollywood, J.A., Lee, C.M., Scallan, M.F. & Harrison, P.T. Analysis of gene repair tracts from Cas9/gRNA double-stranded breaks in the human CFTR gene. Sci. Rep. 6, 32230 (2016).
Hubbard, N. et al. Targeted gene editing restores regulated CD40L function in X-linked hyper-IgM syndrome. Blood 127, 2513–2522 (2016).
Grieger, J.C., Choi, V.W. & Samulski, R.J. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1, 1412–1428 (2006).
Gregorevic, P. et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 10, 828–834 (2004).
Aurnhammer, C. et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods 23, 18–28 (2012).
Lock, M. et al. Characterization of a recombinant adeno-associated virus type 2 reference standard material. Hum. Gene Ther. 21, 1273–1285 (2010).
Park, C.Y., Majeti, R. & Weissman, I.L. In vivo evaluation of human hematopoiesis through xenotransplantation of purified hematopoietic stem cells from umbilical cord blood. Nat. Protoc. 3, 1932–1940 (2008).
Notta, F., Doulatov, S. & Dick, J.E. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood 115, 3704–3707 (2010).
Chicaybam, L., Sodre, A.L., Curzio, B.A. & Bonamino, M.H. An efficient low cost method for gene transfer to T lymphocytes. PLoS One 8, e60298 (2013).
Danet, G.H., Pan, Y., Luongo, J.L., Bonnet, D.A. & Simon, M.C. Expansion of human SCID-repopulating cells under hypoxic conditions. J. Clin. Invest. 112, 126–135 (2003).
Koller, M.R., Bender, J.G., Papoutsakis, E.T. & Miller, W.M. Effects of synergistic cytokine combinations, low oxygen, and irradiated stroma on the expansion of human cord blood progenitors. Blood 80, 403–411 (1992).
Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. Appendix 3, Appendix 3B; http://dx.doi.org/10.1002/0471142735.ima03bs21 (2001).
Reinisch, A. et al. A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat. Med. 22, 812–821 (2016).
Psaila, B. et al. Single-cell profiling of human megakaryocyte-erythroid progenitors identifies distinct megakaryocyte and erythroid differentiation pathways. Genome Biol. 17, 83 (2016).
Bak, R.O. et al. Multiplexed genetic engineering of human hematopoietic stem and progenitor cells using CRISPR/Cas9 and AAV6. Elife 6, e27873 (2017).
Acknowledgements
R.O.B. was supported through an individual postdoctoral grant (DFF-1333-00106B) and a Sapere Aude, Research Talent grant (DFF-1331-00735B), both from the Danish Council for Independent Research, Medical Sciences. D.P.D. was supported through a Stanford Child Health Research Institute (CHRI) grant and postdoctoral award. M.H.P. gratefully acknowledges the support of the Amon Carter Foundation, the Laurie Kraus Lacob Faculty Scholar Award in Pediatric Translational Research, and NIH grants R01-AI097320 and R01-AI120766. We further thank members of the Porteus and Majeti lab for helpful input, comments, and discussions.
Author information
Authors and Affiliations
Contributions
R.O.B. and D.P.D. contributed equally to this work, as well as designed and performed most of the experiments necessary for the methodological development presented here. M.H.P. directed the research and participated in the design and interpretation of the experiments and the writing of the protocol. R.O.B. and D.P.D. wrote the manuscript with help from M.H.P.
Corresponding author
Ethics declarations
Competing interests
M.H.P. is a consultant and has equity interest in CRISPR Tx, but CRISPR Tx had no input into the design, execution, interpretation, or publication of the methods or results herein.
Integrated supplementary information
Supplementary Figure 1 Gating scheme for identifying and quantifying the number of HSPCs with targeted integration of a GFP reporter gene.
FACS plots show the gating strategy for identifying CD34+ HSPCs targeted with a GFP reporter gene. The figure shows data from cord blood-derived CD34+ HSPCs that were electroporated two days after isolation with Cas9 RNP targeting the HBB locus. Immediately after electroporation, cells were transduced at an MOI of 50,000 using an AAV6 donor vector carrying a UbC-GFP expression cassette. Four days after electroporation and transduction, cells were stained for CD34 (and propidium iodide to identify live cells) and then analyzed on a FACS Aria (BD). Cells are identified in a forward/side scatter plot (FCS-A and SSC-A) and single cells discriminated from doublets using SSC-W/SSC-H and FSC-W/FSC-H plots. Live cells are discriminated based on propidium iodide, and in the final GFP/CD34 plot, the targeted GFP+CD34+ cells are identified. Cell frequencies within gates are shown.
Supplementary Figure 2 Gating strategy to identify stem and progenitor subpopulations within the CD34+ cells.
Representative FACS plots are shown from the analysis of CD34+ cells freshly isolated from cord blood. Cells were stained for human lineage markers (CD2, CD3, CD4, CD8, CD16, CD19, CD20, CD56, CD235a, and CD14), as well as CD34, CD38, CD90, CD45RA, and CD123. The gating strategy identifies cells in a forward/side scatter plot (FCS-A and SSC-A) and then single cells are discriminated from doublets using SSC-W/SSC-H and FSC-W/FSC-H plots. Live cells are discriminated based on propidium iodide. Subpopulations are shown in red gates and identified as follows: HSCs (Lin-/CD34+/CD38−/CD45RA−/CD90+), MPPs (Lin−/CD34+/CD38−/CD45RA−/CD90−), LMPPs (Lin−, CD34+, CD38−, CD90−, CD45RA+), CMPs (Lin−/CD34+/CD38+/CD45RA−/CD123+), GMPs (Lin−/CD34+/CD38+/CD45RA+/CD123+), and MEPs (Lin−/CD34+/CD38+/CD45RA−/CD123−). HSCs: hematopoietic stem cells, MPPs: multipotent progenitors, LMPPs: lymphoid-primed multipotent progenitors, CMPs: common myeloid progenitors, GMPs: granulocyte-macrophage progenitors, MEPs: megakaryocyte-erythrocyte progenitors.
Supplementary Figure 3 Gating strategy for analyzing human engraftment in NSG mice.
Representative FACS plots show the gating strategy upstream of the FACS plots shown in Figure 4D. Cells are identified in a forward/side scatter plot (FCS-A and SSC-A) and single cells discriminated from doublets using SSC-W/SSC-H and FSC-W/FSC-H plots. Live cells and murine red blood cells (RBCs) are discriminated based on propidium iodide and an anti-murine Ter119 antibody. Out of the live non-RBCs, human cells can be gated based on huCD45 and HLA-ABC expression as depicted in Figure 4D. Adapted with permission from ref. 15, Springer Nature.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and the Supplementary Methods. (PDF 1826 kb)
Rights and permissions
About this article
Cite this article
Bak, R., Dever, D. & Porteus, M. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat Protoc 13, 358–376 (2018). https://doi.org/10.1038/nprot.2017.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.143
This article is cited by
-
Transient inhibition of 53BP1 increases the frequency of targeted integration in human hematopoietic stem and progenitor cells
Nature Communications (2024)
-
Genome engineering with Cas9 and AAV repair templates generates frequent concatemeric insertions of viral vectors
Nature Biotechnology (2024)
-
Enrichment strategies to enhance genome editing
Journal of Biomedical Science (2023)
-
Efficient delivery of a large-size Cas9-EGFP vector in porcine fetal fibroblasts using a Lonza 4D-Nucleofector system
BMC Biotechnology (2023)
-
Stable expression of large transgenes via the knock-in of an integrase-deficient lentivirus
Nature Biomedical Engineering (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.