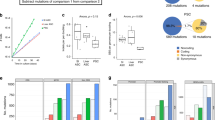
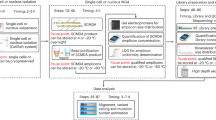
Abstract
Characterization of mutational processes in adult stem cells (ASCs) will improve our understanding of aging-related diseases, such as cancer and organ failure, and may ultimately help prevent the development of these diseases. Here, we present a method for cataloging mutations in individual human ASCs without the necessity of using error-prone whole-genome amplification. Single ASCs are expanded in vitro into clonal organoid cultures to generate sufficient DNA for accurate whole-genome sequencing (WGS) analysis. We developed a data-analysis pipeline that identifies with high confidence somatic variants that accumulated in vivo in the original ASC. These genome-wide mutation catalogs are valuable resources for the characterization of the underlying mutational mechanisms. In addition, this protocol can be used to determine the effects of culture conditions or mutagen exposure on mutation accumulation in ASCs in vitro. Here, we describe a protocol for human liver ASCs that can be completed over a period of 3–4 months with hands-on time of ∼5 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blokzijl, F. et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 (2016).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Zhu, L. et al. Multi-organ mapping of cancer risk. Cell 166, 1132–1146.e7 (2016).
Adams, P.D., Jasper, H. & Rudolph, K.L. Aging-induced stem cell mutations as drivers for disease and cancer. Cell Stem Cell 16, 601–612 (2015).
Behjati, S. et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature 513, 422–425 (2014).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Nik-Zainal, S. et al. The life history of 21 breast cancers. Cell 149, 994–1007 (2012).
Helleday, T., Eshtad, S. & Nik-Zainal, S. Mechanisms underlying mutational signatures in human cancers. Nat. Rev. Genet. 15, 585–598 (2014).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Drost, J. et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc. 11, 347–358 (2016).
Boj, S.F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Sun, B., Beicheng, S. & Michael, K. Obesity, inflammation, and liver cancer. J. Hepatol. 56, 704–713 (2012).
Hoeijmakers, J.H.J. DNA damage, aging, and cancer. N. Engl. J. Med. 361, 1475–1485 (2009).
Kuijk, E.W. et al. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci. Rep. 6, 22154 (2016).
Vermeij, W.P. et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 537, 427–431 (2016).
Alexandrov, L.B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Schwank, G. & Clevers, H. CRISPR/Cas9-mediated genome editing of mouse small intestinal organoids. Methods Mol. Biol. 1422, 3–11 (2016).
Alexandrov, L.B. et al. Clock-like mutational processes in human somatic cells. Nat. Genet. 47, 1402–1407 (2015).
Fitzgerald, D.M., Hastings, P.J. & Rosenberg, S.M. Stress-induced mutagenesis: implications in cancer and drug resistance. Annu. Rev. Cancer Biol. 1, 119–140 (2017).
Xie, M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Martincorena, I. et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015).
Schuster-Böckler, B. & Lehner, B. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature 488, 504–507 (2012).
Gawad, C., Koh, W. & Quake, S.R. Single-cell genome sequencing: current state of the science. Nat. Rev. Genet. 17, 175–188 (2016).
Takasato, M., Minoru, T., Er, P.X., Chiu, H.S. & Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 11, 1681–1692 (2016).
Huang, S.X.L. et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 32, 84–91 (2014).
Ronen, D. & Benvenisty, N. Genomic stability in reprogramming. Curr. Opin. Genet. Dev. 22, 444–449 (2012).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Bartfeld, S. et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126–136.e6 (2015).
Kessler, M. et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 6, 8989 (2015).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Watanabe, K. et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686 (2007).
van Heesch, S. et al. Systematic biases in DNA copy number originate from isolation procedures. Genome Biol. 14, R33 (2013).
Boeva, V. et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics 28, 423–425 (2012).
Van der Auwera, G.A. et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1–11.10. (2013).
Sherry, S.T. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 29, 308–311 (2001).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Tarasov, A., Vilella, A.J., Cuppen, E., Nijman, I.J. & Prins, P. Sambamba: fast processing of NGS alignment formats. Bioinformatics 31, 2032–2034 (2015).
Gentleman, R. R Programming for Bioinformatics (CRC Press, 2008).
Quinlan, A.R. BEDTools: The Swiss-Army tool for genome feature analysis. Curr. Protoc. Bioinformatics 47, 11.12.1–11.12. (2014).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Derrien, T. et al. Fast computation and applications of genome mappability. PLoS One 7, e30377 (2012).
Blokzijl, F., Janssen, R., Van Boxtel, R. & Cuppen, E. MutationalPatterns: comprehensive genome-wide analysis of mutational processes. Preprint at bioRxivhttp://doi.org/10.1101/071761 (2017).
Alexandrov, L.B., Nik-Zainal, S., Wedge, D.C., Campbell, P.J. & Stratton, M.R. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 3, 246–259 (2013).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
Rausch, T. et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333–i339 (2012).
Huddleston, J. et al. Discovery and genotyping of structural variation from long-read haploid genome sequence data. Genome Res. 27, 677–685 (2016).
Chen, L., Liu, P., Evans, T.C. Jr. & Ettwiller, L.M. DNA damage is a pervasive cause of sequencing errors, directly confounding variant identification. Science 355, 752–756 (2017).
Smith, K.S. et al. SomVarIUS: somatic variant identification from unpaired tissue samples. Bioinformatics 32, 808–813 (2016).
Acknowledgements
The authors thank J. de Ligt for his input on the CNV analysis, and J.F. van Velzen for his input on the FACS procedures. This study was financially supported by a Zenith grant from the Netherlands Genomics Initiative (935.12.003) and funding from the NWO Zwaartekracht program Cancer Genomics.nl to E.C., and funding from Worldwide Cancer Research (WCR, grant no. 16-0193) to R.v.B.
Author information
Authors and Affiliations
Contributions
M.J., F.B., H.C., R.v.B. and E.C. wrote the manuscript. M.J., R.v.B. and V.S. developed the wet lab protocol, and N.B. tested the protocol. The bioinformatics pipeline was developed by F.B. and R.v.B., implemented by F.B. and tested by S.B. and R.J.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Outgrowth potential for organoid formation after picking clonal organoids.
Each dot represents a single human donor. There is no correlation between the age of the human donor and the number of picked organoids that were expanded (correlation = -0.041, p-value = 0.482).
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 and Supplementary Table 1. (PDF 307 kb)
Rights and permissions
About this article
Cite this article
Jager, M., Blokzijl, F., Sasselli, V. et al. Measuring mutation accumulation in single human adult stem cells by whole-genome sequencing of organoid cultures. Nat Protoc 13, 59–78 (2018). https://doi.org/10.1038/nprot.2017.111
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.111
This article is cited by
-
Initiation of Cancer: The Journey From Mutations in Somatic Cells to Epigenetic Changes in Tissue-resident VSELs
Stem Cell Reviews and Reports (2024)
-
Widespread somatic L1 retrotransposition in normal colorectal epithelium
Nature (2023)
-
Grave-to-cradle: human embryonic lineage tracing from the postmortem body
Experimental & Molecular Medicine (2023)
-
One-step generation of tumor models by base editor multiplexing in adult stem cell-derived organoids
Nature Communications (2023)
-
Common anti-cancer therapies induce somatic mutations in stem cells of healthy tissue
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.