Abstract
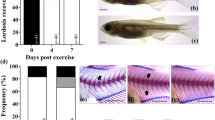
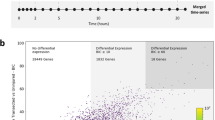
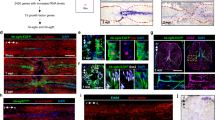
Here we present a protocol for the husbandry of Xenopus laevis tadpoles and froglets, and procedures to study spinal cord regeneration. This includes methods to induce spinal cord injury (SCI); DNA and morpholino electroporation for genetic studies; in vivo imaging for cell analysis; a swimming test to measure functional recovery; and a convenient model for screening for new compounds that promote neural regeneration. These protocols establish X. laevis as a unique model organism for understanding spinal cord regeneration by comparing regenerative and nonregenerative stages. This protocol can be used to understand the molecular and cellular mechanisms involved in nervous system regeneration, including neural stem and progenitor cell (NSPC) proliferation and neurogenesis, extrinsic and intrinsic mechanisms involved in axon regeneration, glial response and scar formation, and trophic factors. For experienced personnel, husbandry takes 1–2 months; SCI can be achieved in 5–15 min; and swimming recovery takes 20–30 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thuret, S., Moon, L. & Gage, F. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628–643 (2006).
Lee-Liu, D., Edwards-Faret, G., Tapia, V.S. & Larraín, J. Spinal cord regeneration: lessons for mammals from non-mammalian vertebrates. Genesis 51, 529–544 (2013).
Diaz Quiroz, J.F. & Echeverri, K. Spinal cord regeneration: where fish, frogs and salamanders lead the way, can we follow? Biochem. J. 451, 353–364 (2013).
Sims, R.T. Transection of the spinal cord in developing Xenopus laevis. J. Embryol. Exp. Morphol. 10, 115–126 (1962).
Michel, M.E. & Reier, P.J. Axonal-ependymal associations during early regeneration of the transected spinal cord in Xenopus laevis tadpoles. J. Neurocytol. 8, 529–548 (1979).
Filoni, S., Bosco, L. & Cioni, C. Reconstitution of the spinal cord after ablation in larval Xenopus laevis. Acta Embryol. Morphol. Exp. 5, 109–129 (1984).
Beattie, M.S., Bresnahan, J.C. & Lopate, G. Metamorphosis alters the response to spinal cord transection in Xenopus laevis frogs. J. Neurobiol. 21, 1108–1122 (1990).
Gibbs, K.M., Chittur, S.V. & Szaro, B.G. Metamorphosis and the regenerative capacity of spinal cord axons in Xenopus laevis. Eur. J. Neurosci. 33, 9–25 (2011).
Gaete, M. et al. Spinal cord regeneration in Xenopus tadpole proceeds through activation of Sox2-positive cells. Neural Dev. 7, 13 (2012).
Muñoz, R. et al. Regeneration of Xenopus laevis spinal cord requires Sox2/3 expressing cells. Dev. Biol. 408, 229–243 (2015).
Lee-Liu, D. et al. Genome-wide expression profile of the response to spinal cord injury in Xenopus laevis reveals extensive differences between regenerative and non-regenerative stages. Neural Dev. 9, 12 (2014).
Nieuwkoop, P.D. & Faber, J. Normal Table of Xenopus laevis (Daudin). New York: Garland Publishing, 1994.
Gargioli, C. & Slack, J.M. Cell lineage tracing during Xenopus tail regeneration. Development 131, 2669–2679 (2004).
Love, N.R. et al. Genome-wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Dev. Biol. 11, 70 (2011).
Contreras, E.G., Gaete, M., Sánchez, N., Carrasco, H. & Larraín, J. Early requirement of hyaluronan for tail regeneration in Xenopus tadpoles. Development 136, 2987–2996 (2009).
Lukovic, D. et al. Complete rat spinal cord transection as a faithful model of spinal cord injury for translational cell transplantation. Sci. Rep. 10, 9640 (2015).
Metz, G.A. et al. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J. Neurotrauma 17, 1–17 (2000).
Jakeman, L.B. et al. Traumatic spinal cord injury produced by controlled contusion in mouse. J. Neurotrauma 17, 299–319 (2000).
Nout, Y.S. et al. Animal models of neurologic disorders: a nonhuman primate model of spinal cord injury. Neurotherapeutics 9, 380–392 (2012).
Sharif-Alhoseini, M. & Rahimi-Movaghar, V. Animal Models in Traumatic Spinal Cord Injury, Topics in Paraplegia. (ed. Dionyssiotis Y.) 211–217 (InTech, 2014).
Buchholz, D.R. More similar than you think: frog metamorphosis as a model of human perinatal endocrinology. Dev. Biol. 408, 188–195 (2015).
Gearhart, J., Oster-Granite, M.L. & Guth, L.L. Histological changes after transection of the spinal cord of fetal and neonatal mice. Exp. Neurol. 66, 1–15 (1979).
Becker, T. et al. Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules. J. Neurosci. 18, 5789–5803 (1998).
Reimer, M.M. et al. Motor neuron regeneration in adult zebrafish. J. Neurosci. 28, 8510–8516 (2008).
O'Hara, C.M., Egar, M.W. & Chernoff, E.A. Reorganization of the ependyma during axolotl spinal cord regeneration: changes in intermediate filament and fibronectin expression. Dev. Dyn. 193, 103–115 (1992).
Zukor, K.A., Kent, D.T. & Odelberg, S.J. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 6, 1 (2011).
Chernoff, E.A. Spinal cord regeneration: a phenomenon unique to urodeles? Int. J. Dev. Biol. 40, 823–831 (1996).
Becker, T., Wullimann, M.F., Becker, C.G., Bernhardt, R.R. & Schachner, M. Axonal regrowth after spinal cord transection in adult zebrafish. J. Comp. Neurol. 377, 577–595 (1997).
Kundi, S., Bicknell, R. & Ahmed, Z. Spinal cord injury: current mammalian models. Am. J. Neurosci. 3, 1–12 (2013).
Cheriyan, T. et al. Spinal cord injury models: a review. Spinal Cord 52, 588–595 (2014).
Singh, A., Tetreault, L., Kalsi-Ryan, S., Nouri, A. & Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 6, 309–331 (2014).
Yisheng, W. et al. First aid and treatment for cervical spinal cord injury with fracture and dislocation. Indian J. Orthop. 41, 300–304 (2007).
Nakae, A. et al. The animal model of spinal cord injury as an experimental pain model. J. Biomed. Biotechnol. 2011, 939023 (2011).
Cline, H.T. & Kelly, D. Xenopus as an experimental system for developmental neuroscience: introduction to a special issue. Dev. Neurobiol. 72, 463–464 (2012).
Karpinka, J.B. et al. Xenbase, the Xenopus model organism database; new virtualized system, data types and genomes. Nucleic Acids Res. 43, 756–763 (2015).
James-Zorn, C. et al. Xenbase: core features, data acquisition, and data processing. Genesis 53, 486–497 (2015).
Session, A.M., Kwon, T., Chapman, J.A. et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 538, 336–343 (2016).
Kroll, K.L. & Amaya, E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 173–183 (1996).
Ishibashi, S., Kroll, K.L. & Amaya, E. A method for generating transgenic frog embryos. Methods Mol. Biol. 461, 447–466 (2008).
Harland, R.M. & Grainger, R.M. Xenopus research: metamorphosed by genetics and genomics. Trends Genet. 27, 507–515 (2011).
Blitz, I.L., Biesinger, J., Xie, X. & Cho, K.W. Biallelic genome modification in F(0) Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis 51, 827–834 (2013).
Nakayama, T. et al. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis 51, 835–843 (2013).
Wang, F. et al. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 5, 15 (2015).
Bhattacharya, D., Marfo, C.A., Li, D., Lane, M. & Khokha, M.K. CRISPR/Cas9: an inexpensive, efficient loss of function tool to screen human disease genes in Xenopus. Dev. Biol. 408, 30269–30264 (2015).
McKeown, C.R., Sharma, P., Sharipov, H.E., Shen, W. & Cline, H.T. Neurogenesis is required for behavioral recovery after injury in the visual system of Xenopus laevis. J. Comp. Neurol. 521, 2262–2278 (2013).
Bestman, J.E., Ewald, R.C., Chiu, S.L. & Cline, H.T. In vivo single-cell electroporation for transfer of DNA and macromolecules. Nat. Protoc. 1, 1267–1272 (2006).
Sive, H.L., Grainger, R.M. & Harland, R.M. Early Development of Xenopus laevis: A Laboratory Manual. (eds. Sive, H.L., Grainger, R.M., Harland, R.M.) 4–5 (Cold Spring Harbor, NY: Cold Spring Harbor Press, 2000).
Green, S.L. The Laboratory Xenopus sp. 19–24 (Boca Raton, FL: Taylor & Francis Group/CRC Press, 2010).
Javaherian, A. & Cline, H.T. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron 17, 505–512 (2005).
Diaz-Quiroz, J.F. & Echeverri, K. In vivo modulation of microRNA levels during spinal cord regeneration. Lab. Methods Cell Biol. Vol. 112 (ed. Coon, M.) 235–246 (Elsevier, 2012).
Tanaka, EM. & Ferretti, P. Considering the evolution of regeneration in the central nervous system. Nat. Rev. Neurosci. 10, 713–723 (2009).
Fei, J.F. et al. CRISPR-mediated genomic deletion of Sox2 in the axolotl shows a requirement in spinal cord neural stem cell amplification during tail regeneration. Stem Cell Rep. 3, 444–459 (2014).
Ogai, K. et al. Function of Sox2 in ependymal cells of lesioned spinal cords in adult zebrafish. Neurosci. Res. 88, 84–87 (2014).
Nye, H.L. & Cameron, J.A. Strategies to reduce variation in Xenopus regeneration studies. Dev. Dyn. 234, 151–158 (2005).
Acknowledgements
We thank members of our laboratory for their support, and especially our dedicated animal caretaker A. Farias. We thank H.T. Cline (The Scripps Research Institute) for our collaborative work with two-photon microscopy. This work was supported by funds from the CARE Chile UC-Centro de Envejecimiento y Regeneración (PFB 12/2007), MINREB (RC120003), FONDECYT (1141162), and ICGEB (CRP/CHI-13-01), FONDEF Idea (ID15I10349) to J.L. and Gastos Operacionales (21110043) to G.E.-F. G.E.-F., D.L.-L. and E.E.M.-O. are CONICYT PhD fellows.
Author information
Authors and Affiliations
Contributions
G.E.-F., R.M., E.E.M.-O., D.L.-.L. and V.S.T. contributed to developing and improving the protocols described here. G.E.-F. prepared the figures and videos. G.E.-F. and J.L. conceived and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Spinal cord injury in Xenopus laevis larvae.
Video explains surgery to induce spinal cord injury and the control sham surgery in larvae (Steps 10–12). (AVI 26179 kb)
Spinal cord injury in Xenopus laevis froglets.
Video explains surgery to induce spinal cord injury and the control sham surgery in froglets (Steps 16–20). (AVI 20720 kb)
Intracoelomic injections in Xenopus laevis larvae.
Video explains the intracoelomic injection of small molecules into larvae (Steps 25A(viii) and Box 1). (AVI 6844 kb)
Intracoelomic injections in Xenopus laevis froglets.
Video explains the intracoelomic injection of small molecules into froglets (Box 1). (AVI 6214 kb)
Spinal cord dissection for whole-mount immunofluorescence.
Video explains larva spinal cord dissection for whole-mount immunofluorescence (Steps 25A(xi–xvi)). (AVI 22272 kb)
Spinal cord isolation from Xenopus laevis larvae.
Video explains spinal cord isolation for -omics analysis in larvae (Box 3, step 4A). (AVI 17736 kb)
Spinal cord isolation from Xenopus laevis froglets.
Video explains spinal cord isolation for -omics analysis in froglets (Box 3, step 4B). (AVI 23323 kb)
Rights and permissions
About this article
Cite this article
Edwards-Faret, G., Muñoz, R., Méndez-Olivos, E. et al. Spinal cord regeneration in Xenopus laevis. Nat Protoc 12, 372–389 (2017). https://doi.org/10.1038/nprot.2016.177
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.177
This article is cited by
-
Cellular response to spinal cord injury in regenerative and non-regenerative stages in Xenopus laevis
Neural Development (2021)
-
Fosl1 is vital to heart regeneration upon apex resection in adult Xenopus tropicalis
npj Regenerative Medicine (2021)
-
Analysis of the early response to spinal cord injury identified a key role for mTORC1 signaling in the activation of neural stem progenitor cells
npj Regenerative Medicine (2021)
-
Temporally distinct transcriptional regulation of myocyte dedifferentiation and Myofiber growth during muscle regeneration
BMC Genomics (2017)
-
The brain is required for normal muscle and nerve patterning during early Xenopus development
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.