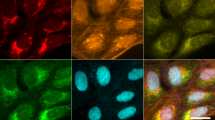
Abstract
Mitochondria have a central role in cellular (patho)physiology, and they display a highly variable morphology that is probably coupled to their functional state. Here we present a protocol that allows unbiased and automated quantification of mitochondrial 'morphofunction' (i.e., morphology and membrane potential), cellular parameters (size, confluence) and nuclear parameters (number, morphology) in intact living primary human skin fibroblasts (PHSFs). Cells are cultured in 96-well plates and stained with tetramethyl rhodamine methyl ester (TMRM), calcein–AM (acetoxy-methyl ester) and Hoechst 33258. Next, multispectral fluorescence images are acquired using automated microscopy and processed to extract 44 descriptors. Subsequently, the descriptor data are subjected to a quality control (QC) algorithm based upon principal component analysis (PCA) and interpreted using univariate, bivariate and multivariate analysis. The protocol requires a time investment of ∼4 h distributed over 2 d. Although it is specifically developed for PHSFs, which are widely used in preclinical research, the protocol is portable to other cell types and can be scaled up for implementation in high-content screening.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Chandel, N.S. Mitochondria as signalling organelles. BMC Biol. 12, 34 (2014).
Westermann, B. Mitochondrial fission and fusion in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872–884 (2010).
Chan, D.C. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 256–287 (2012).
Twig, G. et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446 (2008).
Willems, P.H.G.M., Rossignol, R., Dieteren, C.E.J., Murphy, M.P. & Koopman, W.J.H. Redox regulation and mitochondrial dynamics. Cell Metab. 22, 207–218 (2015).
Mitchell, P. & Moyle, J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature 213, 137–179 (1967).
Koopman, W.J.H., Distelmaier, F., Esseling, J.J., Smeitink, J.A.M. & Willems, P.H.G.M. Computer-assisted live cell analysis of mitochondrial membrane potential, morphology and calcium handling. Methods 46, 304–311 (2008).
Iannetti, E. et al. High-content and high-throughput analysis of mitochondrial dynamics. Int. J. Biochem. Cell Biol. 63, 66–70 (2015).
Mishra, P. & Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 212, 379–387.
Schrepfer, E. & Scorrano, L. Mitofusins, from mitochondria to metabolism. Mol. Cell 61, 683–694 (2016).
Toyama, E.Q. et al. AMPK-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351, 275–281 (2016).
Wai, T. & Langer, T. Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metab. 27, 105–117 (2016).
Koopman, W.J.H., Willems, P.H.G.M. & Smeitink, J.A.M. Monogenic mitochondrial disorders. N. Eng. J. Med. 366, 1132–1141 (2012).
Koopman, W.J.H., Distelmaier, F., Smeitink, J.A.M. & Willems, P.H.G.M. OXPHOS mutations and neurodegeneration. EMBO J. 32, 9–29 (2013).
Tronstad, K.J. et al. Regulation and quantification of cellular mitochondrial morphology and content. Curr. Pharm. Des. 20, 5643–5652 (2014).
Rowland, A.A. & Voeltz, G.K. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat. Rev. Mol. Cell Biol. 13, 607–625 (2012).
Picard, M., Shirihai, O.S., Gentil, B.J. & Burelle, Y. Mitochondrial morphology transitions and functions: implications for retrograde signalling? Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R393–R406 (2013).
Koopman, W.J.H. et al. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid. Redox Signal. 12, 1431–1470 (2010).
Koopman, W.J.H. et al. Partial complex I inhibition decreases mitochondrial motility and increases matrix protein diffusion as revealed by fluorescence correlation spectroscopy. Biochim. Biophys. Acta 1767, 940–947 (2007).
Koopman, W.J.H. et al. Inhibition of complex I of the electron transport chain causes oxygen radical-mediated mitochondrial outgrowth. Am. J. Physiol. Cell Physiol. 288, C1440–C1450 (2005).
Koopman, W.J.H. et al. Mitochondrial network complexity and pathological decrease in complex I activity are tightly correlated in isolated human complex I deficiency. Am. J. Physiol. Cell Physiol. 289, C881–C890 (2005).
Koopman, W.J.H., Visch, H.J., Smeitink, J.A.M. & Willems, P.H.G.M. Simultaneous, quantitative measurement and automated analysis of mitochondrial morphology, mass, potential and motility in living human skin fibroblasts. Cytometry A 69A, 1–12 (2006).
Distelmaier, F. et al. Life cell quantification of mitochondrial membrane potential at the single organelle level. Cytometry A 73, 129–138 (2008).
Willems, P.H.G.M., Smeitink, J.A.M. & Koopman, W.J.H. Mitochondrial dynamics in human NADH:oxidoreductase deficiency. Int. J. Biochem. Cell Biol. 41, 1773–1783 (2009).
Nooteboom, M., Forkink, M., Willems, P.H.G.M. & Koopman, W.J.H. Live-cell quantification of mitochondrial functional parameters. in Neuromethods 70: Visualization Techniques, from Immunohistochemistry to Magnetic Resonance Imaging (ed. Badoer, E.) Chapter 6 (Springer, New York, 2012).
Blanchet, L., Buydens, L.M.C., Smeitink, J.A.M., Willems, P.H.G.M. & Koopman, W.J.H. Isolated mitochondrial complex I deficiency: explorative pattern analysis of patient cell parameters. Curr. Pharm. Des. 17, 4023–4033 (2011).
Blanchet, L. et al. Analysis of small molecule phenotypic effects using combined mitochondrial morpho-functional fingerprinting and machine learning. Sci. Rep. 5, 8035 (2015).
Koopman, W.J.H. et al. Human NADH: oxidoreductase deficiency: radical changes in mitochondrial morphology? Am. J. Physiol. Cell Physiol. 293, C22–C29 (2007).
Koopman, W.J.H. et al. Inherited complex I deficiency is associated with faster protein diffusion in the matrix of moving mitochondria. Am. J. Physiol. Cell Physiol. 294, C1124–C1132 (2008).
Lansing-Taylor, D. Past, present and future of high content screening and the field of cellomics. in High Content Screening: A Powerful Approach to Systems Cell Biology and Drug Discovery (eds. Lansing-Taylor, D., Haskins, J.R. & Giuliano, K.A.) (Humana Press, Totowa, NJ, USA, 2007).
Feng, Y., Mitchison, T.J., Bender, A., Young, D.W. & Tallarico, J.A. Multi-parameter phenotypic profiling: using cellular effects to characterize small-molecule compounds. Nat. Rev. Drug Discov. 8, 567–578 (2009).
Zanella, F., Lorens, J.B. & Link, W. High content screening: seeing is believing. Trends Biotechnol. 28, 237–245 (2010).
Bast, A. & Haenen, G.R.M.M. Ten misconceptions about antioxidants. Trends Pharmacol. Sci. 34, 430–436 (2013).
Wiemerslage, L. & Lee, D. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J. Neurosci. Methods 262, 56–65 (2016).
Sommer, C. & Gehrlich, D.W. Machine learning in cell biology – teaching computers to recognize phenotypes. J. Cell Sci. 126, 5529–5539 (2013).
Peng, J.Y. et al. Automatic morphological subtyping reveals new roles of caspases in mitochondrial dynamics. PLoS Comput. Biol. 7, e1002212 (2011).
Reis, Y. et al. Multi-parametric analysis and modeling of relationships between mitochondrial morphology and apoptosis. PLoS One 7, e28694 (2012).
Ahmad, T. et al. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 4, e461 (2013).
Leonard, A.P. et al. Quantitative analysis of mitochondrial morphology and membrane potential in living cells using high-content imaging, machine learning, and morphological binning. Biochim. Biophys. Acta 1853, 348–360 (2015).
Nikolaisen, J. et al. Automated quantification and integrative analysis of 2D and 3D mitochondrial shape and network properties. PLoS One 9, e101365 (2014).
Farrand, L. et al. An improved quantitative approach for the assessment of mitochondrial fragmentation in chemoresistant ovarian cancer cells. PLoS One 8, e74008 (2014).
Rizk, A. et al. Segmentation and quantification of subcellular structures in fluorescence microscopy images using Squassh. Nat. Protoc. 9, 586–596 (2014).
Lautenschläger, J. et al. Novel computer vision algorithm for the reliable analysis of organelle morphology in whole cell 3D images - A pilot study for the quantitative evaluation of mitochondrial fragmentation in amyotrophic lateral sclerosis. Mitochondrion 25, 49–59 (2015).
Lihavainen, E., Kislin, M., Toptunov, D., Khiroug, L. & Ribeiro, A.S. Automatic quantification of mitochondrial fragmentation from two-photon microscope images of mouse brain tissue. J. Microsc. 260, 338–351 (2015).
Hugelier, S. et al. Sparse deconvolution of high-density super-resolution images. Sci. Rep. 6, 21413 (2016).
McClatchey, P.M., Keller, A.C., Bouchard, R., Knaub, L.A. & Reusch, J.E.B. Fully automated software for quantitative measurements of mitochondrial morphology. Mitochondrion 26, 58–71 (2016).
Giuly, R.J., Martone, M.E. & Ellisman, M.H. Method: automatic segmentation of mitochondria utilizing patch classification, contour pair classification, and automatically seeded level sets. BMC Bioinformatics 13, 29 (2012).
Lihavainen, E., Makela, J., Spelbrink, J.N. & Ribeiro, A.S. Mitoe: automatic analysis of mitochondrial dynamics. Bioinformatics 28, 1050–1051 (2012).
Mumcuoglu, E.U. et al. Computerized detection and segmentation of mitochondria on electron microscope images. J. Microsc. 246, 248–265 (2012).
Dietlmaier, J., Ghita, O., Duessmann, H., Prehn, J.H. & Whelan, P.F. Unsupervised mitochondria segmentation using recursive spectral clustering and adaptive similarity models. J. Struct. Biol. 184, 401–408 (2013).
Bros, H., Hauser, A., Niesner, R. & Infante-Duarte, C. Assessing mitochondrial movement within neurons: manual versus automated tracking methods. Traffic 16, 906–917 (2015).
Kandel, J., Chou, P. & Eckmann, D.M. Automated detection of whole-cell mitochondrial motility and its dependence on cytoarchitectural integrity. Biotechnol. Bioeng. 112, 1395–1405 (2015).
Tasel, S.F., Mumcuoglu, E.U., Hassanpour, R.Z. & Perkins, G. A validated active contour method driven by parabolic arc model for detection and segmentation of mitochondria. J. Struct. Biol. 194, 253–271 (2016).
Forkink, M. et al. Mitochondrial hyperpolarization during chronic complex I inhibition is sustained by low activity of complex II, III, IV and V. Biochim. Biophys. Acta 1837, 1247–1256 (2014).
Nicholls, D.G. Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J. Biol. Chem. 281, 14864–14874 (2006).
Hüser, J., Rechenmacher, C.E. & Blatter, L.A. Imaging the permeability transition in single mitochondria. Biophys. J. 74, 2129–2137 (1998).
Falchi, A.M., Isola, R., Diana, A., Putzolu, M. & Diaz, G. Characterization of depolarization and repolarization phases of mitochondrial membrane potential fluctuations induced by tetramethylrhodamine methyl ester photoactivation. FEBS J. 272, 1649–1659 (2005).
Blanchet, L., Grefte, S., Smeitink, J.A.M., Willems, P.H.G.M. & Koopman, W.J.H. Photo-induction and automated quantification of reversible mitochondrial permeability transition pore opening in primary mouse myotubes. PLoS One 9, e114090 (2014).
Wang, X.M. et al. A new microcellular cytotoxicity test based upon calcein AM release. Hum. Immunol. 37, 264–270 (1993).
Homolya, L. et al. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J. Biol. Chem. 268, 21496–21496 (1993).
Sternberg, S.R. Biomedical image processing. Computer 16, 22–34 (1983).
Russ, J.C. & Neal, F.B. The Image Processing Handbook 7th edn. (CRC press, Boca Raton, FL, 2016).
Bray, M.A. & Carpenter, A. Advanced assay development guidelines for image-based high content screening and analysis. in Assay Guidance Manual (eds. Sittampalam, G.S. et al.) (Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda, MD, 2004).
Tigges, J. et al. The hallmarks of fibroblast aging. Mech. Ageing Dev. 138, 26–44 (2014).
Massart, D.L. et al. Handbook of Chemometrics and Qualimetrics (Amsterdam, the Netherlands: Elsevier, 1997).
Tukey, J.W. Exploratory Data Analysis 1st edn. (Addison-Wesley, Boston, MA, 1977).
Ringnér, M. What is principal component analysis? Nat. Biotechol. 26, 303–304 (2008).
Bro, R. & Smilde, A.K. Principal component analysis. Anal. Methods 6, 2812–2831 (2014).
Brown, J.D. Choosing the right number of components of factors in PCA and EFA. Shiken: JALT testing & evaluation SIG Newsletter 13(2), 19–23 (2009).
Lemasters, J.J. & Ramshesh, V.K. Imaging of mitochondrial polarization and depolarization with cationic fluorophores. Methods Cell. Biol. 80, 283–295 (2007).
Eliceiri, K.W. et al. Biological imaging software tools. Nat. Methods 9, 697–710 (2012).
Kitami, T. et al. A chemical screen probing the relationship between mitochondrial content and cell size. PLoS One 7, e33755 (2012).
Gonzalez, R.C. & Woods, R.E. Digital Image Processing 3rd edn. (Pearson Prentice Hall, Upper Saddle River, NJ, 2008).
Acknowledgements
This research was supported by the Marie-Curie Initial Training Networks (ITN) grant 'MEET' (Mitochondrial European Educational Training; FP7-PEOPLE-2012-ITN), a PM-Rare (Priority Medicines Rare Disorders and Orphan Diseases) grant from the Netherlands Organization for Health Research and Development—Medical Sciences (40-41900-98-033), theEnergy4All Foundation (http://www.energy4all.eu) and the CSBR (Centres for Systems Biology Research) initiative from the Netherlands Organisation for Scientific Research (NWO; CSBR09/013V). We thank L. Blanchet and M. Pellegrini (both from Khondrion BV) for training, practical assistance and discussions.
Author information
Authors and Affiliations
Contributions
W.J.H.K. developed the original microscopy and image processing strategy on which this protocol is based. E.F.I., J.B. and W.J.H.K. designed the experiments. E.F.I. performed the experiments. E.F.I. and W.J.H.K. analyzed the data and prepared the figures. E.F.I., J.A.M.S., J.B., P.H.G.M.W. and W.J.H.K. wrote and proof-read the manuscript. W.J.H.K. supervised the research.
Corresponding author
Ethics declarations
Competing interests
This research was carried out in a collaborative project of the Department of Biochemistry (Radboud University Medical Center) and Khondrion BV (a Radboud University Medical Center spin-off biotech company). E.F.I. (full time), J.B. (full time) and J.A.M.S. (part time) are employed by Khondrion BV. P.H.G.M.W. and W.J.H.K. are scientific consultants for Khondrion BV.
Integrated supplementary information
Supplementary Figure 1 Importance of the threshold (T) gray value for correct calculation of the BIN and MSK images.
(A) Typical RAW calcein image (left image) and the effect of various thresholds (numerals depict the threshold gray value) on the resulting binary (BIN) image. (B) Same as panel A but now for the RAW TMRM image of the same cells. (C) TMRM MSK image calculated using the BIN images in panel B. (D) Average value of Casum (total area of the objects in the calcein BIN image) and Asum (total area of the mitochondrial objects in the TMRM MSK image) for various threshold values. (E) Average value of AR (aspect ratio of mitochondrial objects), F (form factor: length and degree of branching of mitochondrial objects) and Mm (mitochondrial mass) in the TMRM MSK image for various threshold values. (F) Average value of Dm (mitochondrial TMRM intensity in TMRM MSK image), Ot (the total number of mitochondrial objects in the TMRM MSK image), Am (the area of individual mitochondrial objects in the TMRM MSK image) and Nc (the number of mitochondria per cell; calculated by combining information from the TMRM MSK and Hoechst BIN image) for various threshold values. Using a threshold value of 40 yielded the correct values of key descriptors between 3-6 (F), 2-3 (AR) and 50-150 (Nc), compatible with previous manual analysis (e.g. Koopman et al., 2005a; Koopman et al., 2005b; Koopman et al., 2006; Koopman et al., 2008a; Distelmaier et al., 2008; Distelmaier et al., 2012; Distelmaier et al., 2015).
Supplementary Figure 2 Illustration of too low and too high cell density.
Since images are automatically acquired for each well there is no user-control regarding which cells are imaged. Although this strategy avoids user-induced bias, it requires that the average number of imaged cells is similar between individual wells. The latter is also important to allow standardized image processing, statistical analysis and QC. This figure provides typical examples of too low and too high cell densities (relative to the proper density depicted in Figure 5). The protocol described in the main text was used to acquire the images in this figure.
Supplementary Figure 3 Importance of the presence of extracellular TMRM, well mixing and illumination intensity for TMRM descriptor analysis.
This figure illustrates why it is important to have TMRM present outside the cell during image TMRM acquisition, why wells need to be properly mixed following TMRM addition, and the artefact-inducing effect of too high illumination intensities. Unless stated otherwise, the experimental data in this figure was acquired using the experimental protocol described in the main text. (A) Importance of the presence of extracellular TMRM during TMRM image acquisition. In this experiment the calcein/Hoechst loading and imaging steps were omitted. Three different manoeuvres were tested (using 5 plates/days for each condition), thereby modifying step 15 of the protocol as follows: “Washing” condition (gray symbols; two washing steps were performed using 100 µl assay medium; 100 µl of assay medium was present in each well during image acquisition); “No washing” condition (open symbols; nothing was done to the plate after step 13); “Dilution” condition (black symbols; 100 µl assay medium was added to each well without washing and mixing). Only for the last condition, the fluorescence signal remained stable as indicated by the slope (inset) of a linear fit (red line). All data were normalized to the average of the first row (row A) of the plate. Fitting results: P<0.001, R=-0.83 (Washing condition); P<0.001, R=0.91 (No washing condition); P=0.029, R=-0.22 (Dilution condition). (B) Importance of mixing after TMRM addition to the well. The following steps of the protocol were modified: Step 13 (TMRM was added and mixed by pipetting up and down 4 times, but only in row A and B. For row C to H, TMRM was added but not mixed. Calcein/Hoechst staining and imaging were not required here. Only in the mixed wells (green), the TMRM signal was stable (i.e. the fitted line had no significant slope). (C) Effect of TMRM illumination intensity on the TMRM fluorescence signal. In this experiment, 30 subsequent images of the same well were acquired at the same position (Option in BD AttoVision Software: image acquisition option/acquire button/30 data points. Three different acquisition protocols were used with the following settings: Protocol 1 (Exposure time: 0.1 s; Excitation B: 548/20; Excitation dichroic: 40%; Lamp B intensity: 60%), protocol 2 (Exposure time: 0.1 s; Excitation B: 548/20; Excitation dichroic: open; Lamp B intensity: 80%), protocol 3 (Exposure time: 0.1 s; Excitation B: 548/20; Excitation dichroic: open; Lamp B intensity: 100%). Acquisition protocol 1 (used in the protocol) displayed the lowest drop in TMRM signal. Fitting results: TMRM intensity curves were fitted using a mono-exponential equation (y=y0+A1·e-t/τ, with τ being the decay time constant). The fitted τ values equalled: 44.0±23.4 (acquisition protocol 1), 17.4±0.384 (acquisition protocol 2) and 7.56±0.129 (acquisition protocol 3). (D) Effect of the three illumination protocols on the calculated mitochondrial area (Am) and number of mitochondrial objects per cell (Nc). The more the TMRM signal drops, the greater the erroneous apparent increase in Am and Nc. (E) Similar to panel D but now for the calculated mitochondrial aspect ratio (AR) and form factor (F). (F) Values of calculated key mitochondrial descriptors (Nc, Am, AR and F; y-axis) as a function of TMRM intensity (x-axis) for acquisition protocol 1. About 13 illuminations can be carried out (i.e. 13 images can be acquired) before the (10-15%) drop in TMRM intensity affects descriptor quantification.
Supplementary Figure 4 Illustration of the effect of image defocusing on TMRM-reported mitochondrial morphology descriptors.
It is of the greatest importance that the acquired images are optimally focused, since defocusing will affect the extracted numerical data in a descriptor-dependent manner. This figures illustrates the effects of image defocusing on key mitochondrial descriptors. Typically, defocused images contain “artificial” small non-mitochondrial objects after image processing. The latter results in an apparent increase in the number of mitochondrial objects per cell (Nc), and a decrease in mitochondrial size (Am) and form factor (F). Statistics: Significant differences between the focused and defocused condition were assessed using an unpaired independent Student’s test and presented by: * (P<0.05), the actual P-value (Am) and by non-significant (n.s.). The protocol described in the main text was used to acquire the images in this figure.
Supplementary Figure 5 Control for TMRM autoquenching, detectability of Δψ hyperpolarization and effect of the mitochondrial uncoupler FCCP.
This figure illustrates how to check if the TMRM fluorescence signal is affected by autoquenching and whether Δψ depolarization and hyperpolarization can be detected by decreased and increased mitochondrial TMRM accumulation, respectively. (A) Eighty-five consecutive TMRM images were acquired from the same well. The displayed typical image-pairs illustrate the effect of the mitochondrial uncoupler p-trifluoromethoxy carbonyl cyanide phenyl hydrazine (FCCP, 10 µM; #370-86-5; Sigma-Aldrich, St. Louis, MO, USA) on mitochondrial TMRM staining (upper vs. lower panel). Numerals mark individual cells and regions of interest (ROIs; yellow) mark the nuclear and mitochondrial compartment (yellow circles). (B) Average ratio between TMRM intensity in the mitochondrial and nuclear compartments calculated using the ROIs in panel A for untreated (n=4 cells) and FCCP-treated (n=2) cells. Repeated illumination induces a gradual drop in ratio (photobleaching) in the untreated cells. FCCP induces a smooth drop in the ratio signal, indicating the absence of TMRM autoquenching. If the latter would be the case, FCCP would first induce an increase in the signal (due to TMRM dequenching in the mitochondrial matrix), followed by a decrease. (C) Effect of subsequent acute addition of the FoF1-ATPase inhibitor Oligomycin A (OLI; 1 µM; #1404-19-9; Sigma-Aldrich), the mitochondrial complex I inhibitor Rotenone (ROT; 1 µM; #83-79-4; Sigma-Aldrich) and FCCP (10 µM) on mitochondrial TMRM fluorescence intensity. In a second type of experiment, Bonkrekic acid (BA; 50 µM; #11076-19-0; Sigma-Aldrich) was acutely added to inhibit the electrogenic mitochondrial ATP/ADP translocator (ANT). This was followed by subsequent addition of ROT and FCCP. Statistics: Significant differences with the indicated columns (a,b,c,d,e,f) was assessed using an unpaired independent Student’s test (Origin Pro 6.1) and presented by: *** (P<0.001). Experimental details: Options used in the BD attovision software: “Montage Capture Setup”, “INACTIVE MONTAGE”, “1 Frame”. MACRO on BD attovision software: Experiment 1 (30 (CT) TMRM image acquisitions of the same well; 30 s pause during which 1 µM Oligomycin (OLI) is added by pipetting; 30 TMRM image acquisitions of the same well; 30 s pause during which 1 µM Rotenone (ROT) is added; 30 TMRM image acquisitions of the same well; 30 s pause during which 10 µM FCCP is added; 30 TMRM image acquisitions of the same well); Experiment 2 (30 (CT) TMRM image acquisitions of the same well; 30 s pause during which 50 µM Bongkrekic acid (BA) is added; 30 TMRM image acquisitions of the same well; 30 s pause during which 1 µM Rotenone (ROT) is added; 30 TMRM image acquisitions of the same well; 30 s pause during with 10 µM FCCP is added; 30 TMRM image acquisitions of the same well). Remarks: Care should be taken that during addition of the inhibitors the pipette tip does not touch the plate to avoid altering the field of view. Inhibitors are added at twice their final concentration in a (relatively large) volume of 100 µl to each well (which contains 100 µl of fluid). This ensures complete and rapid mixing.
Supplementary Figure 6 Visualization of the effect of median (MED) and top-hat (THF) spatial filtering on the TMRM image.
Proper use of the MED and THF filters to reduce noise and isolate mitochondrial objects, respectively, is crucial for proper subsequent calculation of the binary image (BIN). The latter image represents white mitochondrial objects on a black background (for the complete processing pipeline see Fig. 5C). In this protocol, we have established the correct size of the MED and THF filter, how often they should be applied (“passes”) and, in case of the THF filter, its strength (“S”). For details about these parameters see the information in Box 1 provided in the main text. (A) Effect of various MED filter settings on the TMRM image (RAW). (B) Effect of various THF filter settings on the TMRM image (RAW).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–3 and Supplementary References (PDF 1914 kb)
Supplementary Software
Dataset for IPP and MATLAB analysis (ZIP 5018 kb)
Rights and permissions
About this article
Cite this article
Iannetti, E., Smeitink, J., Beyrath, J. et al. Multiplexed high-content analysis of mitochondrial morphofunction using live-cell microscopy. Nat Protoc 11, 1693–1710 (2016). https://doi.org/10.1038/nprot.2016.094
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.094
This article is cited by
-
A time window for rescuing dying retinal ganglion cells
Cell Communication and Signaling (2024)
-
Mitochondrial heterogeneity in diseases
Signal Transduction and Targeted Therapy (2023)
-
Metabolic impact of genetic and chemical ADP/ATP carrier inhibition in renal proximal tubule epithelial cells
Archives of Toxicology (2023)
-
A novel automated image analysis pipeline for quantifying morphological changes to the endoplasmic reticulum in cultured human cells
BMC Bioinformatics (2021)
-
SK channel-mediated metabolic escape to glycolysis inhibits ferroptosis and supports stress resistance in C. elegans
Cell Death & Disease (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.