Abstract
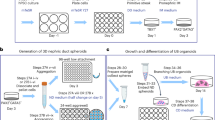
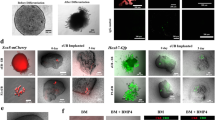
This protocol presents recently developed methodologies for the differentiation of human pluripotent stem cells (hPSCs) into ureteric bud (UB) progenitor–like cells. Differentiation of human PSCs to UB progenitor–like cells allows for the generation of chimeric kidney cultures in which the human cells can self-assemble into chimeric 3D structures in combination with embryonic mouse kidney cells over a period of 18 d. UB progenitor–like cells are generated by a two-step process that combines in vitro commitment of human PSCs, whether embryonic stem cells (ESCs) or induced PSCs (iPSCs), under chemically defined culture conditions, with ex vivo cultures for the induction of 3D organogenesis. The models described here provide new opportunities for investigating human kidney development, modeling disease, evaluating regenerative medicine strategies, as well as for toxicology studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Takebe, T. et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat. Protoc. 9, 396–409 (2014).
Lancaster, M.A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Hockemeyer, D. et al. Genetic engineering of human pluripotent cells using TALE nucleases. Nat. Biotechnol. 29, 731–734 (2011).
Shen, B. et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat. Methods 11, 399–402 (2014).
Liu, G.H. et al. Targeted gene correction of laminopathy-associated LMNA mutations in patient-specific iPSCs. Cell Stem Cell 8, 688–694 (2011).
Xia, Y. et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat. Cell Biol. 15, 1507–1515 (2013).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Takasato, M. et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118–126 (2014).
Raya, A. et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460, 53–59 (2009).
Kabgani, N. et al. Primary cultures of glomerular parietal epithelial cells or podocytes with proven origin. PLoS ONE 7, e34907 (2012).
Shankland, S.J., Pippin, J.W., Reiser, J. & Mundel, P. Podocytes in culture: past, present, and future. Kidney Int. 72, 26–36 (2007).
Little, M.H. & McMahon, A.P. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb. Perspect. Biol. 4, pii: a008300 (2012).
Batchelder, C.A., Lee, C.C., Matsell, D.G., Yoder, M.C. & Tarantal, A.F. Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation 78, 45–56 (2009).
Vigneau, C. et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J. Am. Soc. Nephrol. 18, 1709–1720 (2007).
Bruce, S.J. et al. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation 75, 337–349 (2007).
Kim, D. & Dressler, G.R. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J. Am. Soc. Nephrol. 16, 3527–3534 (2005).
Davies, J.A., Unbekandt, M., Ineson, J., Lusis, M. & Little, M.H. Dissociation of embryonic kidney followed by re-aggregation as a method for chimeric analysis. Methods Mol. Biol. 886, 135–146 (2012).
Unbekandt, M. & Davies, J.A. Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int. 77, 407–416 (2010).
Chang, C.H. & Davies, J.A. An improved method of renal tissue engineering, by combining renal dissociation and reaggregation with a low-volume culture technique, results in development of engineered kidneys complete with loops of Henle. Nephron Exp. Nephrol. 121, e79–85 (2012).
Sebinger, D.D. et al. A novel, low-volume method for organ culture of embryonic kidneys that allows development of cortico-medullary anatomical organization. PLoS ONE 5, e10550 (2010).
Amit, M. & Itskovitz-Eldor, J. Atlas of Human Pluripotent Stem Cells (Humana Press, 2012).
Costantini, F., Watanabe, T., Lu, B., Chi, X. & Srinivas, S. Dissection of embryonic mouse kidney, culture in vitro, and imaging of the developing organ. Cold Spring Harb. Protoc. 2011 10.1101/pdb.prot5613 (2011).
Ludwig, T.E. et al. Feeder-independent culture of human embryonic stem cells. Nat. Methods 3, 637–646 (2006).
Acknowledgements
We thank M. Schwarz for administrative support. We thank J. Kasuboski from the Waitt Advanced Biophotonics Core at the Salk Institute for Biological Studies for help with imaging processing. Y.X. was partially supported by the California Institute for Regenerative Medicine (CIRM) through a CIRM Training grant. I.S.-M. was partially supported by a Nomis Foundation postdoctoral fellowship. Work in the laboratory of J.C.I.B. was supported by grants from the G. Harold and Leila Y. Mathers Charitable Foundation and The Leona M. and Harry B. Helmsley Charitable Trust (2012-PG-MED002).
Author information
Authors and Affiliations
Contributions
Y.X., I.S.-M., E.N., C.R.E., J.M.C. and J.C.I.B. designed all experiments and developed the methodologies presented here. Y.X., I.S.-M., E.N. and J.C.I.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xia, Y., Sancho-Martinez, I., Nivet, E. et al. The generation of kidney organoids by differentiation of human pluripotent cells to ureteric bud progenitor–like cells. Nat Protoc 9, 2693–2704 (2014). https://doi.org/10.1038/nprot.2014.182
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.182
This article is cited by
-
Precision nephrotoxicity testing using 3D in vitro models
Cell & Bioscience (2023)
-
Human reconstructed kidney models
In Vitro Cellular & Developmental Biology - Animal (2021)
-
3D kidney organoids for bench-to-bedside translation
Journal of Molecular Medicine (2021)
-
Concise review: current trends on applications of stem cells in diabetic nephropathy
Cell Death & Disease (2020)
-
Directed Differentiation of Human Pluripotent Stem Cells to Podocytes under Defined Conditions
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.