Abstract
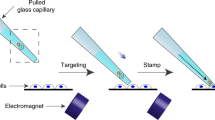
This microinjection protocol allows the manipulation and tracking of neural stem and progenitor cells in tissue at single-cell resolution. We demonstrate how to apply microinjection to organotypic brain slices obtained from mice and ferrets; however, our technique is not limited to mouse and ferret embryos, but provides a means of introducing a wide variety of membrane-impermeable molecules (e.g., nucleic acids, proteins, hydrophilic compounds) into neural stem and progenitor cells of any developing mammalian brain. Microinjection experiments are conducted by using a phase-contrast microscope equipped with epifluorescence, a transjector and a micromanipulator. The procedure normally takes ∼2 h for an experienced researcher, and the entire protocol, including tissue processing, can be performed within 1 week. Thus, microinjection is a unique and versatile method for changing and tracking the fate of a cell in organotypic slice culture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Costa, M.R. et al. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development 138, 1057–1068 (2011).
Saito, K. et al. Morphological asymmetry in dividing retinal progenitor cells. Dev. Growth Differ. 45, 219–229 (2003).
Miyata, T. et al. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133–3145 (2004).
Takahashi, M., Sato, K., Nomura, T. & Osumi, N. Manipulating gene expressions by electroporation in the developing brain of mammalian embryos. Differentiation 70, 155–162 (2002).
Calegari, F., Haubensak, W., Yang, D., Huttner, W.B. & Buchholz, F. Tissue-specific RNA interference in postimplantation mouse embryos with endoribonuclease-prepared short interfering RNA. Proc. Natl. Acad. Sci. USA 99, 14236–14240 (2002).
Molotkov, D.A., Yukin, A.Y., Afzalov, R.A. & Khiroug, L.S. Gene delivery to postnatal rat brain by non-ventricular plasmid injection and electroporation. J. Vis. Exp. 43, 2244 (2010).
Borrell, V. In vivo gene delivery to the postnatal ferret cerebral cortex by DNA electroporation. J. Neurosci. Methods 186, 186–195 (2010).
Kawasaki, H., Toda, T. & Tanno, K. In vivo genetic manipulation of cortical progenitors in gyrencephalic carnivores using in utero electroporation. Biol. Open 2, 95–100 (2013).
Kawasaki, H., Iwai, L. & Tanno, K. Rapid and efficient genetic manipulation of gyrencephalic carnivores using in utero electroporation. Mol. Brain 5, 24 (2012).
Sessa, A., Mao, C.A., Hadjantonakis, A.K., Klein, W.H. & Broccoli, V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron 60, 56–69 (2008).
Farkas, L.M. et al. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron 60, 40–55 (2008).
Fietz, S.A. & Huttner, W.B. Cortical progenitor expansion, self-renewal and neurogenesis—a polarized perspective. Curr. Opin. Neurobiol. 21, 23–35 (2011).
Lui, J.H., Hansen, D.V. & Kriegstein, A.R. Development and evolution of the human neocortex. Cell 146, 18–36 (2011).
Borrell, V. & Reillo, I. Emerging roles of neural stem cells in cerebral cortex development and evolution. Dev. Neurobiol. 72, 955–971 (2012).
Rosa, P. et al. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J. Cell Biol. 109, 17–34 (1989).
Pepperkok, R., Saffrich, R. & Ansorge, W. Computer-automated capillary microinjection of macromolecules into living cells. In Cell Biology: A Laboratory Handbook, Vol. 3 (ed. Celis, J.E.) 22–30 (Academic Press, 1994).
Taverna, E., Haffner, C., Pepperkok, R. & Huttner, W.B. A new approach to manipulate the fate of single neural stem cells in tissue. Nat. Neurosci. 15, 329–337 (2012).
Götz, M. & Huttner, W.B. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788 (2005).
Echeverri, K., Clarke, J.D. & Tanaka, E.M. In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev. Biol. 236, 151–164 (2001).
Echeverri, K. & Tanaka, E.M. Electroporation as a tool to study in vivo spinal cord regeneration. Dev. Dyn. 226, 418–425 (2003).
Arai, Y. et al. Neural stem and progenitor cells shorten S-phase on commitment to neuron production. Nat. Commun. 2, 154 (2011).
Takahashi, T., Nowakowski, R.S. & Caviness, V.S. Jr. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 15, 6046–6057 (1995).
Reillo, I. & Borrell, V. Germinal zones in the developing cerebral cortex of ferret: ontogeny, cell cycle kinetics, and diversity of progenitors. Cereb. Cortex 22, 2039–2054 (2012).
Lukaszewicz, A. et al. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 47, 353–364 (2005).
Haubensak, W., Attardo, A., Denk, W. & Huttner, W.B. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA 101, 3196–3201 (2004).
Miyata, T., Kawaguchi, A., Saito, K., Kuramochi, H. & Ogawa, M. Visualization of cell cycling by an improvement in slice culture methods. J. Neurosci. Res. 69, 861–868 (2002).
Ansorge, W. & Pepperkok, R. Performance of an automated system for capillary microinjection into living cells. J. Biochem. Biophys. Meth. 16, 283–292 (1988).
Pulvers, J.N. & Huttner, W.B. Brca1 is required for embryonic development of the mouse cerebral cortex to normal size by preventing apoptosis of early neural progenitors. Development 136, 1859–1868 (2009).
Minobe, S. et al. Rac is involved in the interkinetic nuclear migration of cortical progenitor cells. Neurosci. Res. 63, 294–301 (2009).
Chen, L., Melendez, J., Campbell, K., Kuan, C.Y. & Zheng, Y. Rac1 deficiency in the forebrain results in neural progenitor reduction and microcephaly. Dev. Biol. 325, 162–170 (2009).
Leone, D.P., Srinivasan, K., Brakebusch, C. & McConnell, S.K. The rho GTPase Rac1 is required for proliferation and survival of progenitors in the developing forebrain. Dev. Neurobiol. 70, 659–678 (2010).
Miyata, T., Kawaguchi, A., Okano, H. & Ogawa, M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727–741 (2001).
Konno, D. et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 10, 93–101 (2008).
Kosodo, Y. et al. Cytokinesis of neuroepithelial cells can divide their basal process before anaphase. EMBO J. 27, 3151–3163 (2008).
Acknowledgements
We thank J. Helppi and other members of the animal facility as well as H. Wolf of the workshop and K. Margitudis of the photo laboratory of the Max Planck Institute of Molecular Cell Biology and Genetics for excellent support; the staff of BioCrea, especially B. Langen, for ferret care and housing; M. Turrero-García for advice with ferret slice culture; F. Mora-Bermúdez for acquiring and processing the photographs of the microinjection equipment (Fig. 3a and Supplementary Fig. 1a,b); and Y.J. Chang, E. Lewitus and M. Wilsch-Bräuninger for helpful comments on the manuscript. W.B.H. was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (SFB 655, A2; TRR 83, Tp6) and the European Research Council (250197), by the DFG-funded Center for Regenerative Therapies Dresden, and by the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Contributions
F.K.W. and E.T. designed and performed all microinjections and most other experimental work, analyzed the data and wrote the manuscript; C.H. performed experiments; E.T. and W.B.H. supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Microinjection pipette optimization.
Comparison between bad and good microinjection pipette. A good microinjection pipette has longer taper (a, b, arrows) and smaller tip (c, note the smaller angle), as compared to a bad one.
Supplementary Figure 2 Collagen embedding.
Comparison between optimal and suboptimal collagen embedding. Top row: photographs of glass-bottom Petri dishes containing organotypic slices embedded with too little, an optimal amount, or too much collagen. Bottom row: diagram illustrating optimal versus suboptimal collagen embedding (side view of Petri dish). Note the difference in the amount of collagen solution. All animal studies were conducted in accordance with the German animal welfare legislation.
Supplementary information
Supplementary Figure 1
Microinjection pipette optimization. (PDF 3869 kb)
Supplementary Figure 2
Collagen embedding. (PDF 38091 kb)
Rights and permissions
About this article
Cite this article
Wong, F., Haffner, C., Huttner, W. et al. Microinjection of membrane-impermeable molecules into single neural stem cells in brain tissue. Nat Protoc 9, 1170–1182 (2014). https://doi.org/10.1038/nprot.2014.074
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.074
This article is cited by
-
Direct metabolomics for plant cells by live single-cell mass spectrometry
Nature Protocols (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.