Abstract
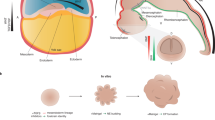
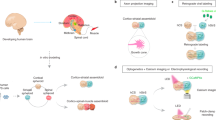
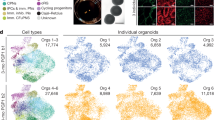
Cerebral organoids, or brain organoids, can be generated from a wide array of emerging technologies for modeling brain development and disease. The fact that they are cultured in vitro makes them easily accessible both genetically and for live assays such as fluorescence imaging. In this Protocol Extension, we describe a modified version of our original protocol (published in 2014) that can be used to reliably generate cerebral organoids of a telencephalic identity and maintain long-term viability for later stages of neural development, including axon outgrowth and neuronal maturation. The method builds upon earlier cerebral organoid methodology, with modifications of embryoid body size and shape to increase surface area and slice culture to maintain nutrient and oxygen access to the interior regions of the organoid, enabling long-term culture. We also describe approaches for introducing exogenous plasmid constructs and for sparse cell labeling to image neuronal axon outgrowth and maturation over time. Together, these methods allow for modeling of later events in cortical development, which are important for neurodevelopmental disease modeling. The protocols described can be easily performed by an experimenter with stem cell culture experience and take 2–3 months to complete, with long-term maturation occurring over several months.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data generated or analyzed during the development of this protocol are included in the figures.
References
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Paşca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Kadoshima, T. et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl Acad. Sci. USA. 110, 20284–20289 (2013).
Zhang, S.-C., Wernig, M., Duncan, I. D., Brüstle, O. & Thomson, J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129–1133 (2001).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Lancaster, M. A. & Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9, 2329–2340 (2014).
Cederquist, G. Y. et al. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 37, 436–444 (2019).
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl Acad. Sci. USA. 112, 15672–15677 (2015).
Bhaduri, A. et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature 578, 142–148 (2020).
Tanaka, Y., Cakir, B., Xiang, Y., Sullivan, G. J. & Park, I.-H. Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 30, 1682–1689.e3 (2020).
Velasco, S. et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527 (2019).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017).
Giandomenico, S. L. et al. Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 22, 669–679 (2019).
Trujillo, C. A. et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell 25, 558–569 (2019).
Lancaster, M. A. et al. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 35, 659–666 (2017).
Jones, E. G. & Rakic, P. Radial columns in cortical architecture: it is the composition that counts. Cereb. Cortex 20, 2261–2264 (2010).
Halfter, W., Dong, S., Yip, Y.-P., Willem, M. & Mayer, U. A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 22, 6029–6040 (2002).
Nasu, M. et al. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS ONE 7, e53024 (2012).
Daza, R. A. M., Englund, C. & Hevner, R. F. Organotypic slice culture of embryonic brain tissue. CSH Protoc. 2007, pdb.prot4914 (2007).
Croft, C. L. & Noble, W. Preparation of organotypic brain slice cultures for the study of Alzheimer’s disease. F1000Res. 7, 592 (2018).
Paşca, S. P. Assembling human brain organoids. Science 363, 126–127 (2019).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Kelava, I. & Lancaster, M. A. Stem cell models of human brain development. Cell Stem Cell 18, 736–748 (2016).
Jo, J. et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257 (2016).
Watanabe, M. et al. Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep. 21, 517–532 (2017).
Cakir, B. et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175 (2019).
Pham, M. T. et al. Generation of human vascularized brain organoids. Neuroreport 29, 588–593 (2018).
Mansour, A. A. et al. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 36, 432–441 (2018).
Miller, F. D. & Gauthier, A. S. Timing is everything: making neurons versus glia in the developing cortex. Neuron 54, 357–369 (2007).
Acknowledgements
The authors thank members of the Lancaster lab for helpful discussions, especially I. Kelava, as well as Z. Neuburger for photos of the ALI-CO procedure. We also thank the LMB light microscopy facility for assistance with imaging. Work in the Lancaster lab is supported by the Medical Research Council (MC_UP_1201/9) and the European Research Council (ERC STG 757710).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.A.L. is an inventor on two cerebral organoid patents with licensing agreements with third parties, including STEMCELL Technologies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature Protocols thanks Wieland Huttner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Related links
Key references using this protocol
Lancaster, M. A. & Knoblich, J. A. Nat. Protoc. 9, 2329–2340 (2014): https://doi.org/10.1038/nprot.2014.158
Lancaster, M. A. et al. Nat. Biotechnol. 35, 659–666 (2017): https://doi.org/10.1038/nbt.3906
Giandomenico, S. L. et al. Nat. Neurosci. 22, 669–679 (2019): https://doi.org/10.1038/s41593-019-0350-2
This protocol is an extension to Nat. Protoc. 9, 2329–2340 (2014): https://doi.org/10.1038/nprot.2014.158
Rights and permissions
About this article
Cite this article
Giandomenico, S.L., Sutcliffe, M. & Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat Protoc 16, 579–602 (2021). https://doi.org/10.1038/s41596-020-00433-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00433-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.