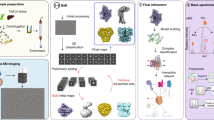
Abstract
Solid-state NMR spectroscopy has been used successfully for characterizing the structure and dynamics of membrane proteins as well as their interactions with other proteins in lipid bilayers. Such an environment is often necessary for achieving native-like structures. Sample preparation is the key to this success. Here we present a detailed description of a robust protocol that results in high-quality membrane protein samples for both magic-angle spinning and oriented-sample solid-state NMR. The procedure is demonstrated using two proteins: CrgA (two transmembrane helices) and Rv1861 (three transmembrane helices), both from Mycobacterium tuberculosis. The success of this procedure relies on two points. First, for samples for both types of NMR experiment, the reconstitution of the protein from a detergent environment to an environment in which it is incorporated into liposomes results in 'complete' removal of detergent. Second, for the oriented samples, proper dehydration followed by rehydration of the proteoliposomes is essential. By using this protocol, proteoliposome samples for magic-angle spinning NMR and uniformly aligned samples (orientational mosaicity of <1°) for oriented-sample NMR can be obtained within 10 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Page, R.C., Li, C., Hu, J., Gao, F.P. & Cross, T.A. Lipid bilayers: an essential environment for the understanding of membrane proteins. Magn. Reson. Chem. 45, S2–S11 (2007).
Dong, H., Sharma, M., Zhou, H.X. & Cross, T.A. Glycines: role in α-helical membrane protein structures and a potential indicator of native conformation. Biochemistry 51, 4779–4789 (2012).
Zhou, H.X. & Cross, T.A. Influences of membrane mimetic environments on membrane protein structures. Annu. Rev. Biophys. 42, 361–392 (2013).
Anfinsen, C.B. Principles that govern the folding of protein chains. Science 181, 223–230 (1973).
Korepanova, A. et al. Cloning and expression of multiple integral membrane proteins from Mycobacterium tuberculosis in Escherichia coli. Protein Sci. 14, 148–158 (2005).
Caffrey, M. Crystallizing membrane proteins for structure determination: use of lipidic mesophases. Annu. Rev. Biophys. 38, 29–51 (2009).
Separovic, F., Killian, J.A., Cotten, M., Busath, D.D. & Cross, T.A. Modeling the membrane environment for membrane proteins. Biophys. J. 100, 2073–2074 (2011).
Li, D. et al. Crystal structure of the integral membrane diacylglycerol kinase. Nature 497, 521–524 (2013).
Chou, J.J., Kaufman, J.D., Stahl, S.J., Wingfield, P.T. & Bax, A. Micelle-induced curvature in a water-insoluble HIV-1 Env peptide revealed by NMR dipolar coupling measurement in stretched polyacrylamide gel. J. Am. Chem. Soc. 124, 2450–2451 (2002).
Van Horn, W.D. et al. Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase. Science 324, 1726–1729 (2009).
Griffin, R.G. Dipolar recoupling in MAS spectra of biological solids. Nat. Struct. Biol. 5, 508–512 (1998).
Tang, W., Knox, R.W. & Nevzorov, A.A. A spectroscopic assignment technique for membrane proteins reconstituted in magnetically aligned bicelles. J. Biomol. NMR 54, 307–316 (2012).
Ketchem, R.R., Hu, W., Tian, F. & Cross, T.A. Structure and dynamics from solid state NMR spectroscopy. Structure 2, 699–701 (1994).
Sharma, M. et al. Insight into the mechanism of the influenza A proton channel from a structure in a lipid bilayer. Science 330, 509–512 (2010).
Mote, K.R. et al. Multidimensional oriented solid-state NMR experiments enable the sequential assignment of uniformly 15N labeled integral membrane proteins in magnetically aligned lipid bilayers. Journal of Biomolecular NMR 51, 339–346 (2011).
Nevzorov, A.A. & Opella, S.J. Selective averaging for high-resolution solid-state NMR spectroscopy of aligned samples. J. Magn. Reson. 185, 59–70 (2007).
Can, T.V. et al. Magic angle spinning and oriented sample solid-state NMR structural restraints combine for influenza A M2 protein functional insights. J. Am. Chem. Soc. 134, 9022–9025 (2012).
Murray, D.T., Das, N. & Cross, T.A. Solid-state NMR strategy for characterizing native membrane protein structures. Accounts Chem. Res. 46, 2172–2181 (2013).
Plocinski, P. et al. Mycobacterium tuberculosis CwsA interacts with CrgA and Wag31, and the CrgA-CwsA complex is involved in peptidoglycan synthesis and cell shape determination. J. Bacteriol. 194, 6398–6409 (2012).
Plocinski, P. et al. Characterization of CrgA, a new partner of the Mycobacterium tuberculosis peptidoglycan polymerization complexes. J. Bacteriol. 193, 3246–3256 (2011).
Li, C. et al. Uniformly aligned full-length membrane proteins in liquid crystalline bilayers for structural characterization. J. Am. Chem. Soc. 129, 5304–5305 (2007).
Su, P.C., Si, W., Baker, D.L. & Berger, B.W. High-yield membrane protein expression from E. coli using an engineered outer membrane protein F fusion. Protein Sci. 22, 434–443 (2013).
Grisshammer, R. Understanding recombinant expression of membrane proteins. Curr. Opin. Biotechnol. 17, 337–340 (2006).
Zoonens, M. & Miroux, B. Expression of membrane proteins at the Escherichia coli membrane for structural studies. Methods Mol. Biol. 601, 49–66 (2010).
Hu, J., Qin, H., Gao, F.P. & Cross, T.A. A systematic assessment of mature MBP in membrane protein production: overexpression, membrane targeting and purification. Protein Expr. Purif. 80, 34–40 (2011).
Mouillac, B. & Baneres, J.L. Mammalian membrane receptors expression as inclusion bodies in Escherichia coli. Methods Mol. Biol. 601, 39–48 (2010).
Qin, H. et al. Construction of a series of vectors for high throughput cloning and expression screening of membrane proteins from Mycobacterium tuberculosis. BMC Biotechnol. 8, 51 (2008).
Kiefer, H. et al. Expression of an olfactory receptor in Escherichia coli: purification, reconstitution, and ligand binding. Biochemistry 35, 16077–16084 (1996).
Aslanidis, C. & de Jong, P.J. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 18, 6069–6074 (1990).
Korepanova, A. et al. Expression of membrane proteins from Mycobacterium tuberculosis in Escherichia coli as fusions with maltose binding protein. Protein Expr. Purif. 53, 24–30 (2007).
Seddon, A.M., Curnow, P. & Booth, P.J. Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta 1666, 105–117 (2004).
Rigaud, J.L., Pitard, B. & Levy, D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta 1231, 223–246 (1995).
Rigaud, J.L. & Levy, D. Reconstitution of membrane proteins into liposomes. Methods Enzymol. 372, 65–86 (2003).
Signorell, G.A., Kaufmann, T.C., Kukulski, W., Engel, A. & Remigy, H.W. Controlled 2D crystallization of membrane proteins using methyl-beta-cyclodextrin. J. Struct. Biol. 157, 321–328 (2007).
DeGrip, W.J., VanOostrum, J. & Bovee-Geurts, P.H.M. Selective detergent-extraction from mixed detergent/lipid/protein micelles, using cyclodextrin inclusion compounds: a novel generic approach for the preparation of proteoliposomes. Biochem. J. 330, 667–674 (1998).
Rigaud, J.L., Levy, D., Mosser, G. & Lambert, O. Detergent removal by non-polar polystyrene beads—applications to membrane protein reconstitution and two-dimensional crystallization. Eur. Biophy. J. Biophys. Lett. 27, 305–319 (1998).
Rigaud, J.L., Mosser, G., Lacapere, J.J., Olofsson, A., Levy, D. & Ranck, J.L. Bio-Beads: an efficient strategy for two-dimensional crystallization of membrane proteins. J. Struct. Biol. 118, 226–235 (1997).
Kimura, T. et al. Recombinant cannabinoid type 2 receptor in liposome model activates G protein in response to anionic lipid constituents. J. Biol. Chem. 287, 4076–4087 (2012).
Park, S.H. et al. Optimization of purification and refolding of the human chemokine receptor CXCR1 improves the stability of proteoliposomes for structure determination. Biochim. Biophys. Acta 1818, 584–591 (2012).
van den Brink-van der Laan, E., Killian, J.A. & de Kruijff, B. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta 1666, 275–288 (2004).
Miao, Y.M. et al. M2 proton channel structural validation from full-length protein samples in synthetic bilayers and E. coli membranes. Angew. Chem. Int. Ed. Engl. 51, 8383–8386 (2012).
Cady, S., Wang, T. & Hong, M. Membrane-dependent effects of a cytoplasmic helix on the structure and drug binding of the influenza virus M2 protein. J. Am. Chem. Soc. 133, 11572–11579 (2011).
Bhate, M.P., Wylie, B.J., Tian, L. & McDermott, A.E. Conformational dynamics in the selectivity filter of KcsA in response to potassium ion concentration. J. Mol. Biol. 401, 155–166 (2010).
Peterson, E. et al. Functional reconstitution of influenza A M2(22-62). Biochim. Biophys. Acta 1808, 516–521 (2011).
Nagy, J.K. & Sanders, C.R. Destabilizing mutations promote membrane protein misfolding. Biochemistry 43, 19–25 (2004).
Wallace, B.A., Lees, J.G., Orry, A.J., Lobley, A. & Janes, R.W. Analyses of circular dichroism spectra of membrane proteins. Protein Sci. 12, 875–884 (2003).
Andreas, L.B., Eddy, M.T., Pielak, R.M., Chou, J. & Griffin, R.G. Magic angle spinning NMR investigation of influenza A M2(18-60): support for an allosteric mechanism of inhibition. J. Am. Chem Soc. 132, 10958–10960 (2010).
Ketchem, R.R., Hu, W. & Cross, T.A. High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR. Science 261, 1457–1460 (1993).
De Angelis, A.A. & Opella, S.J. Bicelle samples for solid-state NMR of membrane proteins. Nat. Protoc. 2, 2332–2338 (2007).
Cevc, G. & Marsh, D. Phospholipid Bilayers: Physical Principles and Models Vol. 5 (John Wiley & Sons, 1987).
Baldus, M. Solid-state nuclear magnetic resonance. In Comprehensive Biophysics Vol. 1 (ed. Engleman, E.) 160–181 (Academic Press, 2012).
Lakatos, A., Mors, K. & Glaubitz, C. How to investigate interactions between membrane proteins and ligands by solid-state NMR. Methods Mol. Biol. 914, 65–86 (2012).
Wu, C.H., Ramamoorthy, A. & Opella, S.J. High resolution heteronuclear dipolar solid-state NMR spectroscopy. J. Magn. Reson. A 109, 270–272 (1994).
Wang, J. et al. Imaging membrane protein helical wheels. J. Magn. Reson. 144, 162–167 (2000).
Marassi, F.M. & Opella, S.J.A solid-state NMR index of helical membrane protein structure and topology. J. Magn. Reson. 144, 150–155 (2000).
Murray, D.T., Lu, Y.T., Cross, T.A. & Quine, J.R. Geometry of kinked protein helices from NMR data. J. Magn. Reson. 210, 82–89 (2011).
Opella, S.J. et al. Structures of the M2 channel-lining segments from nicotinic acetylcholine and NMDA receptors by NMR spectroscopy. Nat. Struct. Biol. 6, 374–379 (1999).
Park, S.H., De Angelis, A.A., Nevzorov, A.A., Wu, C.H. & Opella, S.J. Three-dimensional structure of the transmembrane domain of Vpu from HIV-1 in aligned phospholipid bicelles. Biophys. J. 91, 3032–3042 (2006).
Verardi, R., Shi, L., Traaseth, N.J., Walsh, N. & Veglia, G. Structural topology of phospholamban pentamer in lipid bilayers by a hybrid solution and solid-state NMR method. Proc. Natl. Acad. Sci. USA 108, 9101–9106 (2011).
Zech, S.G., Wand, A.J. & McDermott, A.E. Protein structure determination by high-resolution solid-state NMR spectroscopy: application to microcrystalline ubiquitin. J. Am. Chem. Soc. 127, 8618–8626 (2005).
McDermott, A. Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu. Rev. Biophys. 38, 385–403 (2009).
Castellani, F. et al. Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature 420, 98–102 (2002).
Park, S.H. et al. Structure of the chemokine receptor CXCR1 in phospholipid bilayers. Nature 491, 779–783 (2012).
Das, B.B. et al. Structure determination of a membrane protein in proteoliposomes. J. Am. Chem. Soc. 134, 2047–2056 (2012).
Park, S.H., Das, B.B., De Angelis, A.A., Scrima, M. & Opella, S.J. Mechanically, magnetically, and 'rotationally aligned' membrane proteins in phospholipid bilayers give equivalent angular constraints for NMR structure determination. J. Phys. Chem. B 114, 13995–14003 (2010).
Opella, S.J. Structure determination of membrane proteins in their native phospholipid bilayer environment by rotationally aligned solid-state NMR spectroscopy. Accounts Chem. Res. 46, 2145–2153 (2013).
Cady, S.D. et al. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature 463, 689–692 (2010).
Massotte, D. G protein-coupled receptor overexpression with the baculovirus-insect cell system: a tool for structural and functional studies. Biochim. Biophys. Acta 1610, 77–89 (2003).
McCusker, E.C., Bane, S.E., O'Malley, M.A. & Robinson, A.S. Heterologous GPCR expression: a bottleneck to obtaining crystal structures. Biotechnol. Prog. 23, 540–547 (2007).
Shi, L. & Ladizhansky, V. Magic-angle spinning solid-state NMR experiments for structural characterization of proteins. Methods Mol. Biol. 895, 153–165 (2012).
Gor'kov, P.L. et al. Using low-E resonators to reduce RF heating in biological samples for static solid-state NMR up to 900 MHz. J. Magn. Reson. 185, 77–93 (2007).
Quine, J.R. et al. Intensity and mosaic spread analysis from PISEMA tensors in solid-state NMR. J. Magn. Reson. 179, 190–198 (2006).
Page, R.C. et al. Comprehensive evaluation of solution nuclear magnetic resonance spectroscopy sample preparation for helical integral membrane proteins. J. Struct. Funct. Genomics 7, 51–64 (2006).
Acknowledgements
We thank M.W. Davidson at NHMFL and C. Escobar at FSU, IMB, NHMFL for helping with photography. We also thank P.L. Gor'kov for his design of the OS sample holder and the sample transfer base. This work was supported in part by the US National Institutes of Health (grants AI 074805, AI 073891 and AI 023007) and the US National Science Foundation (through Cooperative Agreement 0654118 between the Division of Materials Research and the State of Florida).
Author information
Authors and Affiliations
Contributions
N.D. and D.T.M. performed all the experiments such as membrane protein expression, purification, solid-state NMR sample preparation and new method development. N.D. prepared all the figures. N.D. and T.A.C. wrote the manuscript, D.T.M. provided essential comments. All three authors coordinated to complete this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 12% (wt/vol) SDS-PAGE of CrgA and Rv1861 membrane protein expression and purification steps.
M: Molecular weight marker, L: Whole cell lysate containing inclusion body and membrane fractions, FT: Flow through from nickel column, Washes: two to three consecutive washing steps, Elutions: Protein elution from nickel column. Molecular weights of the proteins are shown by red color arrows.
Supplementary information
Supplementary Figure 1
12% (wt/vol) SDS-PAGE of CrgA and Rv1861 membrane protein expression and purification steps. (PDF 3068 kb)
Supplementary Methods
CrgA and Rv1861 membrane protein expression and purification; Reconstitution and OS sample preparation of 15N uniform labeled gramicidin A protein in DMPC lipid bilayers. (PDF 208 kb)
Rights and permissions
About this article
Cite this article
Das, N., Murray, D. & Cross, T. Lipid bilayer preparations of membrane proteins for oriented and magic-angle spinning solid-state NMR samples. Nat Protoc 8, 2256–2270 (2013). https://doi.org/10.1038/nprot.2013.129
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.129
This article is cited by
-
Characterizing proteins in a native bacterial environment using solid-state NMR spectroscopy
Nature Protocols (2021)
-
Improving the quality of oriented membrane protein spectra using heat-compensated separated local field experiments
Journal of Biomolecular NMR (2019)
-
Tuneable poration: host defense peptides as sequence probes for antimicrobial mechanisms
Scientific Reports (2018)
-
Uniaxial Diffusional Narrowing of NMR Lineshapes for Membrane Proteins Reconstituted in Magnetically Aligned Bicelles and Macrodiscs
Applied Magnetic Resonance (2018)
-
Tuning the photoexcitation response of cyanobacterial Photosystem I via reconstitution into Proteoliposomes
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.