Abstract
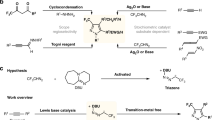
This protocol describes the efficient, generally applicable Ullmann coupling reaction of bromaminic acid with alkyl- or aryl-amines in phosphate buffer under microwave irradiation using elemental copper as a catalyst. The reaction leads to a number of biologically active compounds. As a prototypical example, the synthesis of a new, potent antagonist of human platelet P2Y12 receptors, which has potential as an antithrombotic drug, is described in detail. The optimized protocol includes a description of an appropriate reaction setup, thin layer chromatography for monitoring the reaction and a procedure for the isolation, purification and characterization of the anticipated product. The reaction is performed without the use of a glove box and there is no requirement for an inert atmosphere. The reaction typically proceeds within 2–30 min, the protocol, including workup, generally takes 1–3 h to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Bean, B.P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol. Sci. 13, 87–90 (1992).
Inoue, K. et al. Antagonism by reactive blue 2 but not by brilliant blue G of extracellular ATP-evoked responses in PC12 phaeochromocytoma cells. Br. J. Pharmacol. 102, 851–854 (1991).
Nakazawa, K., Inoue, K., Fujimori, K. & Takanaka, A. Effects of ATP antagonists on purinoceptor-operated inward currents in rat phaeochromocytoma cells. Pflügers Arch. 418, 214–219 (1991).
Brown, J. & Brown, C.A. Evaluation of reactive blue 2 derivatives as selective antagonists for P2Y receptors. Vasc. Pharmacol. 39, 309–315 (2003).
Burnstock, G. Introduction: P2 receptors. Curr. Top. Med. Chem. 4, 793–803 (2004).
Tuluc, F., Bültmann, R., Glänzel, M., Frahm, A.W. & Starke, K. P2-receptor antagonists: IV. Blockade of P2-receptor subtypes and ecto-nucleotidases by compounds related to reactive blue 2. Naunyn Schmiedebergs Arch. Pharmacol. 357, 111–120 (1998).
Ellington, A.E. & Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990).
Schmiedl, J., Damien, S. & Klaus, K. Reactive dyes for dyeing or printing synthetic fibers and their preparation. WO Patent 2004050769 A2 filed 24 Nov. 2003, and issued 17 Jun 2004.
Lauk, U. & Nowack, P. Anthraquinone dyes, their production and their use in color filters. US Patent 2005/0150061 A1 filed 13 Mar. 2003, and issued 14 Jul 2005.
Glänzel, M., Bültmann, R., Starke, K. & Frahm, A.W. Constitutional isomers of Reactive Blue 2—selective P2Y-receptor antagonists? Eur. J. Med. Chem. 38, 303–312 (2003).
Glänzel, M., Bültmann, R., Starke, K. & Frahm, A.W. Structure–activity relationships of novel P2-receptor antagonists structurally related to Reactive Blue 2. Eur. J. Med. Chem. 40, 1262–1276 (2005).
Glänzel, M., Bültmann, R., Starke, K. & Frahm, A.W. Members of the acid blue 129 family as potent and selective P2Y-receptor antagonists. Drug Dev. Res. 59, 64–71 (2003).
Baqi, Y. & Müller, C.E. Rapid and efficient microwave-assisted copper(0)-catalyzed Ullmann coupling reaction: general access to anilinoanthraquinone derivatives. Org. Lett. 9, 1271–1274 (2007).
Weyler, S. et al. Combinatorial synthesis of anilinoanthraquinone derivatives and evaluation as non nucleotide-derived P2Y2 receptor antagonists. Bioorg. Med. Chem. Lett. 18, 223–227 (2008).
Baqi, Y., Atzler, K., Köse, M., Glänzel, M. & Müller, C.E. High-affinity, non nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J. Med. Chem. 52, 3784–3793 (2009).
Baqi, Y., Weyler, S., Iqbal, J., Zimmermann, H. & Müller, C.E. Structure-activity relationships of anthraquinone derivatives derived from bromaminic acid as inhibitors of ectonucleoside triphosphate diphosphohydrolases (E-NTPDases). Purinergic Signal. 5, 91–106 (2009).
Baqi, Y. et al. Development of potent and selective inhibitors of ecto-5′-nucleotidase based on an anthraquinone scaffold. J. Med. Chem. 53, 2076–2086 (2010).
Müller, C.E. P2-pyrimidinergic receptors and their ligands. Curr. Pharm. Des. 8, 2353–2369 (2002).
Brunschweiger, A. & Müller, C.E. P2 receptors activated by uracil nucleotides–an update. Curr. Med. Chem. 13, 289–312 (2006).
Iqbal, J., Vollmayer, P., Braun, N., Zimmermann, H. & Müller, C.E. A capillary electrophoresis method for the characterization of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and the analysis of inhibitors by in-capillary enzymatic microreaction. Purinergic Signal. 1, 349–358 (2005).
Müller, C.E. et al. Polyoxometalates–a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg. Med. Chem. Lett. 16, 5943–5947 (2006).
Ullmann, F. Über eine neue Bildungsweise von Diphenylaminderivaten (On a new formation of diphenylamine derivatives). Chem. Ber. 36, 2382–2384 (1903).
Ullmann, F. Über eine neue Darstellungsweise von Phenyläthersalicylsäure (On a new preparation of phenylethersalicylic acid). Chem. Ber. 37, 853–854 (1904).
Altman, R.A. & Buchwald, S.A. Cu-catalyzed Goldberg and Ullmann reactions of aryl halides using chelating N- and O-based ligands. Nat. Protoc. 2, 2474–2479 (2007).
Pearson, J.C., Burton, S.J. & Lowe, C.R. Affinity precipitation of lactate dehydrogenase with a triazine dye derivative: selective precipitation of rabbit muscle lactate dehydrogenase with a procion blue H-B analog. Anal. Biochem. 158, 382–389 (1986).
Eltz, A. Reactive dyes useful for dyeing and printing e.g. cellulose, polyamide or polyester. DE Patent 4417719 A1 filed 20 May 1994, and issued 23 Nov 1995.
Dallinger, D. & Kappe, C.O. Rapid preparation of the mitotic kinesin Eg5 inhibitor monastrol using controlled microwave-assisted synthesis. Nat. Protoc. 2, 317–321 (2007).
Dallinger, D. & Kappe, C.O. Automated generation of a dihydropyrimidine compound library using microwave-assisted processing. Nat. Protoc. 2, 1713–1721 (2007).
Hayes, B.L. Introduction to microwave chemistry. in Microwave Synthesis, Chemistry at the Speed of Light, 16–28 (CEM Publishing, Matthews, North Carolina, 2002).
Murray, J.K. & Gellman, S.H. Parallel synthesis of peptide libraries using microwave irradiation. Nat. Protoc. 2, 624–631 (2007).
Bacsa, B. & Kappe, C.O. Rapid solid-phase synthesis of a calmodulin binding peptide using controlled microwave irradiation. Nat. Protoc. 2, 2222–2227 (2007).
Guzmán-Mejía, R., Reyes-Rangel, G. & Juaristi, E. Preparation of chiral derivatives of β-Ala containing the α-phenylethyl group: useful starting materials for the asymmetric synthesis of β-amino acids. Nat. Protoc. 2, 2759–2766 (2007).
Li, X. & Danishefsky, S.J. Noncatalytic reaction of isonitriles and carboxylic acids en route to amide-type linkages. Nat. Protoc. 3, 1666–1670 (2008).
Cattaneo, M. Platelet P2 receptors: old and new targets for antithrombotic drugs. Expert. Rev. Cardiovasc. Ther. 5, 45–55 (2007).
Gachet, C. Regulation of platelet functions by P2 receptors. Annu. Rev. Pharmacol. Toxicol. 46, 277–300 (2006).
Hollopeter, G. et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409, 202–207 (2001).
Hoffmann, K. et al. Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J. Pharmacol. Exp. Ther. 331, 648–655 (2009).
Savi, P. et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb. Haemost. 84, 891–896 (2000).
Sugidachi, A., Asai, F., Ogawa, T., Inoue, T. & Koike, H. The in vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. Br. J. Pharmacol. 129, 1439–1446 (2000).
Ingall, A.H. et al. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J. Med. Chem. 42, 213–220 (1999).
Boeynaems, J.M., van Giezen, H., Savi, P. & Herbert, J.M. P2Y receptor antagonists in thrombosis. Curr. Opin. Investig. Drugs 6, 275–282 (2005).
van Giezen, J.J. & Humphries, R.G. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin. Thromb. Hemost. 31, 195–204 (2005).
Müller, C.E. Prodrug approaches for enhancing the bioavailability of drugs with low solubility. Chem. Biodivers. 6, 2071–2083 (2009).
Baqi, Y. & Müller, C.E. Catalyst-free microwave-assisted aminiation of 2-chloro-5-nitrobenzoic acid. J. Org. Chem. 72, 5908–5911 (2007).
Acknowledgements
Y.B. thanks the Deutscher Akademischer Austauschdienst (DAAD) for a PhD Fellowship. Support by the Deutsche Forschungsgemeinschaft (DFG, GRK804) is gratefully acknowledged. We would like to thank Ms. Marion Schneider for providing us with the LC-MS chromatogram.
Author information
Authors and Affiliations
Contributions
Y.B. planned and performed the experiments and prepared the first draft of the manuscript. C.E.M. designed the experiments, supervised the work and finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Baqi, Y., Müller, C. Synthesis of alkyl- and aryl-amino-substituted anthraquinone derivatives by microwave-assisted copper(0)-catalyzed Ullmann coupling reactions. Nat Protoc 5, 945–953 (2010). https://doi.org/10.1038/nprot.2010.63
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.63
This article is cited by
-
Synthesis, characterization and anticancer evaluation of nitrogen-substituted 1-(3-aminoprop-1-ynyl)-4-hydroxyanthraquinone derivatives
Medicinal Chemistry Research (2021)
-
Amorphous Cu0 on Carbon Nanofiber as Recyclable Heterogeneous Catalyst for N-Arylation Reactions
Chemical Research in Chinese Universities (2019)
-
Cu0 Nanoparticles Deposited on Nanoporous Polymers: A Recyclable Heterogeneous Nanocatalyst for Ullmann Coupling of Aryl Halides with Amines in Water
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.