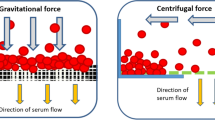
Abstract
In cell affinity chromatography, type-specific cell separation is based on the interaction between cell-surface receptors and an immobilized ligand on a stationary matrix. This protocol describes the preparation of monolithic polyacrylamide and polydimethylacrylamide cryogel affinity matrices that can be used as a generic type-specific cell separation approach. The supermacroporous monolithic cryogel has highly interconnected large pores (up to 100 μm) for convective migration of large particles such as mammalian cells. In this protocol, they are functionalized to immobilize a protein A ligand by a two-step derivatization of epoxy-containing cryogel monolith (reaction with ethylenediamine and glutaraldehyde). Target cells were labeled with specific antibodies and then they were captured in the cryogel through affinity with protein A. These specifically captured cells were recovered in high yields while retaining their viability by mechanical squeezing of the spongy and elastic cryogel matrices. The suggested cell separation protocol takes <30 min for complete separation on a preprepared protein A–immobilized cryogel column.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kumar, A., Galaev, I.Y. & Mattiasson, B. Cell Separation: Fundamentals, Analytical and Preparative Methods (Springer-Verlag Berlin, Heidelberg, 2007).
Shortman, K. Physical procedures for the separation of animal cells. Annu. Rev. Biophys. Bioeng. 1, 93–130 (1972).
Kumar, R.K. & Lykke, A.W.J. Cell separation: a review. Pathology 16, 53–92 (1984).
de Wildt, R.M., van den Hoogen, F.H., van Venrooij, W.J. & Hoet, R.M. Isolation and characterization of single anti-U1A-specific B cells from autoimmune patients. Ann. NY Acad. Sci. 815, 440–442 (1997).
Edelman, G.M., Rutishauser, U. & Millette, C.F. Cell fractionation and arrangement on fibers, beads, and surfaces. Proc. Natl Acad. Sci. USA 68, 2153–2157 (1971).
Givan, A.L. Flow cytometry: an introduction. Methods Mol. Biol. 263, 1–32 (2004).
Herzenberg, L.A., Parks, D., Sahaf, B., Perez, O. & Roederer, M. The history and future of the fluorescence activated cell sorter and flow cytometry: a view from Stanford. Clin. Chem. 48, 1819–1827 (2002).
Hoffman, R.A. & Houck, D.W. Cell separation using flow cytometric cell sorting. In Cell Separation Methods and Applications Vol. 106 (eds. Recktenwald, D. & Radbruch, A.) 237 (Marcel Dekker, New York, 1997).
Bjerke, T., Nielsen, S., Helgestad, J., Nielsen, B.W. & Schiotz, P.O. Purification of human blood basophils by negative selection using immunomagnetic beads. J. Immunol. Methods 157, 49–56 (1993).
Bonanno, G. et al. Clinical isolation and functional characterization of cord blood CD133+ hematopoietic progenitor cells. Transfusion 44, 1087–1097 (2004).
Sonti, S.V. et al. Large scale isolation of expression vector cassette by magnetic triple helix affinity capture. Nucleic Acids Res. 23, 3995–3996 (1995).
Albertson, P.A. Partition of Cell Particles and Macromolecules (Wiley, 1986).
Kumar, A., Kamihira, M., Galaev, I.Y., Mattiasson, B. & Iijima, S. Type-specific separation of animal cells in aqueous two-phase systems using antibody conjugates with temperature-sensitive polymers. Biotechnol. Bioeng. 75, 570–580 (2001).
Clemmitt, R.H. & Chase, H.A. Impact of operating variables on the expanded bed adsorption of Saccharomyces cerevisiae cells using a concanavalin A derivatized perfluorocarbon. Biotechnol. Bioeng. 82, 506–516 (2003).
Ujam, L.B. et al. Isolation of monocytes from human peripheral blood using immuno-affinity expanded-bed adsorption. Biotechnol. Bioeng. 83, 554–566 (2003).
Ghetie, V., Mota, G. & Sjoquist, J. Separation of cells by affinity chromatography on SpA-Sepharose 6mb. J. Immunol. Methods 21, 133–141 (1978).
Feucht, H.E., Hadam, M.R., Frank, F. & Riethmuller, G. Efficient separation of human lymphocytes-T from venous-blood using PVP-coated colloidal silica particles (Percoll). J. Immunol. Methods 38, 43–51 (1980).
Lozinsky, V.I., Plieva, F.M., Galaev, I.Y. & Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 10, 163–188 (2001).
Lozinsky, V.I. et al. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 21, 445–451 (2003).
Kumar, A., Plieva, F.M., Galaev, I.Y. & Mattiasson, B. Affinity fractionation of lymphocytes using a monolithic cryogel. J. Immunol. Methods 283, 185–194 (2003).
Dainiak, M.B., Galaev, I.Y. & Mattiasson, B. Macroporous monolithic hydrogels in a 96-minicolumn plate format for cell surface-analysis and integrated binding/quantification of cells. Enzyme Microb. Technol. 40, 688–695 (2007).
Arvidsson, P. et al. Chromatography of microbial cells using continuous supermacroporous affinity and ion-exchange columns. J. Chromatogr. A 977, 27–38 (2002).
Dainiak, M.B., Plieva, F.M., Galaev, I.Y., Hatti-Kaul, R. & Mattiasson, B. Cell chromatography: separation of different microbial cells using IMAC supermacroporous monolithic columns. Biotechnol. Prog. 21, 644–649 (2005).
Dainiak, M.B., Galaev, I.Y. & Mattiasson, B. Affinity cryogel monoliths for screening for optimal separation conditions and chromatographic separation of cells. J. Chromatogr. A 1123, 145–150 (2006).
Ahlqvist, J. et al. Affinity binding of inclusion bodies on supermacroporous monolithic cryogels using labeling with specific antibodies. J. Biotechnol. 122, 216–225 (2006).
Teilum, A. et al. Binding mitochondria to cryogel monoliths allows detection of proteins specifically released following permeability transition. Anal. Biochem. 348, 209–221 (2006).
Williams, S.L., Eccleston, M.E. & Slater, N.K.H. Affinity capture of a biotinylated retrovirus on macroporous monolithic adsorbents: towards a rapid single-step purification process. Biotechnol. Bioeng. 89, 783–787 (2005).
Kumar, A. et al. Affinity binding of cells to cryogel adsorbents with immobilized specific ligands: effect of ligand coupling and matrix architecture. J. Mol. Recognit. 18, 84–93 (2005).
GE Life Sciences. Ficoll-Paque Plus intended use: for in vitro isolation of lymphocytes. Instructions 71-7167-00 AG (2007).
Acknowledgements
We acknowledge the financial support from the Department of Biotechnology (DBT) and the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India.
Author information
Authors and Affiliations
Contributions
The protocol was jointly conceived and written by both the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kumar, A., Srivastava, A. Cell separation using cryogel-based affinity chromatography. Nat Protoc 5, 1737–1747 (2010). https://doi.org/10.1038/nprot.2010.135
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.135
This article is cited by
-
Penicillium chrysogenum-loaded hybrid cryogel discs for heavy metal removal
Chemical Papers (2023)
-
Efficient immunoaffinity chromatography of lymphocytes directly from whole blood
Scientific Reports (2018)
-
Highly Bactericidal Macroporous Antimicrobial Polymeric Gel for Point-of-Use Water Disinfection
Scientific Reports (2018)
-
Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing
Nature Communications (2018)
-
Superelastic and pH-Responsive Degradable Dendrimer Cryogels Prepared by Cryo-aza-Michael Addition Reaction
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.