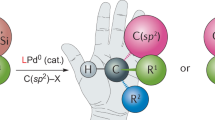
Abstract
The following protocol describes the selective cross-coupling of an amine with an aryl halide using a Pd-based catalyst to provide the corresponding N-arylated amine. This general procedure for C–N bond formation includes a detailed description of an appropriate reaction setup, two methods for assaying the crude reaction mixtures (thin layer chromatography (TLC) and gas chromatography (GC)) and procedures for the isolation, purification and characterization of the anticipated product. Reagents and catalyst precursors can be manipulated in the air; however, the cross-coupling reactions must be performed under an inert atmosphere. Two Pd-catalyzed C–N bond-forming reactions are included in the text as representative examples of these procedures. Although the reactions can proceed in <5 min, the protocols, including workup, generally take 6–30 h to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Fang, Y.Q., Karisch, R. & Lautens, M. Efficient syntheses of KDR kinase inhibitors using a Pd-catalyzed tandem C-N/Suzuki coupling as the key step. J. Org. Chem. 72, 1341–1346 (2007).
Damon, D.B., Dugger, R.W., Hubbs, S.E., Scott, J.M. & Scott, R.W. Asymmetric synthesis of the cholesteryl ester transfer protein inhibitor torcetrapib. Org. Process Res. Dev. 10, 472–480 (2006).
Yandulov, D.V. & Schrock, R.R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
Heaney, H. The benzyne and related intermediates. Chem. Rev. 62, 81–97 (1962).
Smith, M.B. & March, J. March's Advanced Organic Chemistry: Reactions, Mechanisms and Structure 5th edn. (John Wiley & Sons. Inc., New York, 2001).
Belfield, A.J., Brown, G.R. & Foubister, A.J. Recent synthetic advances in the nucleophilic amination of benzenes. Tetrahedron 55, 11399–11428 (1999).
Lindley, J. Copper assisted nucleophilic substitution of aryl halogen. Tetrahedron 40, 1433–1456 (1984).
Beletskaya, I.P. & Cheprakov, A.V. Copper in cross-coupling reactions: the Post-Ullmann chemistry. Coord. Chem. Rev. 248, 2337–2364 (2004).
Ley, S.V. & Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation. Angew. Chem. Int. Ed. Engl. 42, 5400–5449 (2003).
Jiang, L. & Buchwald, S.L. Palladium-catalyzed aromatic carbon-nitrogen bond formation. In Metal-Catalyzed Cross-coupling Reactions 2nd edn. (eds. de Meijere, A. & Diederich, F.) 699–760 (Wiley-VCH, New York, 2004).
Old, D.W., Wolfe, J.P. & Buchwald, S.L. A highly active catalyst for palladium-catalyzed cross-coupling reactions: room-temperature Suzuki couplings and amination of unactivated aryl chlorides. J. Am. Chem. Soc. 120, 9722–9723 (1998).
Huang, X. et al. Expanding Pd-catalyzed C-N bond-forming processes: the first amidation of aryl sulfonates, aqueous amination, and complementarity with Cu-catalyzed reactions. J. Am. Chem. Soc. 125, 6653–6655 (2003).
Wolfe, J.P. & Buchwald, S.L. A highly active catalyst for the room-temperature amination and Suzuki coupling of aryl chlorides. Angew. Chem. Int. Ed. Engl. 38, 2413–2416 (1999).
Anderson, K.W., Tundel, R.E., Ikawa, T., Altman, R.A. & Buchwald, S.L. Monodentate phosphines provide highly active catalysts for Pd-catalyzed C-N bond-forming reactions of heteroaromatic halides/amines and (H)N-heterocycles. Angew. Chem. Int. Ed. Engl. 45, 6523–6527 (2006).
Old, D.W., Harris, M.C. & Buchwald, S.L. Efficient palladium-catalyzed N-arylation of indoles. Org. Lett. 2, 1403–1406 (2000).
Klapars, A., Huang, X. & Buchwald, S.L. A general and efficient copper catalyst for the amidation of aryl halides. J. Am. Chem. Soc. 124, 7421–7428 (2002).
Klapars, A., Antilla, J.C., Huang, X. & Buchwald, S.L. A general and efficient copper catalyst for the amidation of aryl halides and the N-arylation of nitrogen heterocycles. J. Am. Chem. Soc. 123, 7727–7729 (2001).
Kwong, F.Y. & Buchwald, S.L. Mild and efficient copper-catalyzed amination of aryl bromides with primary alkylamines. Org. Lett. 5, 793–796 (2003).
Shafir, A.S. & Buchwald, S.L. Highly selective room-temperature copper-catalyzed C-N coupling reactions. J. Am. Chem. Soc. 128, 8742–8743 (2006).
Antilla, J.C., Klapars, A. & Buchwald, S.L. The copper-catalyzed N-arylation of indoles. J. Am. Chem. Soc. 124, 11684–11688 (2002).
Antilla, J.C., Baskin, J.M., Barder, T.E. & Buchwald, S.L. Copper-diamine-catalyzed N-arylation of pyrroles, pyrazoles, indazoles, imidazoles and triazoles. J. Org. Chem. 69, 5578–5587 (2004).
Altman, R.A. & Buchwald, S.L. 4,7-Dimethoxy-1,10-phenanthroline: an excellent ligand for the Cu-catalyzed N-arylation of imidazoles. Org. Lett. 8, 2779–2782 (2006).
Buchwald, S.L., Mauger, C., Mignani, G. & Scholz, U. Industrial-scale palladium-catalyzed coupling of aryl halides and amines—a personal account. Adv. Synth. Catal. 348, 23–39 (2006).
Anderson, K.W., Ikawa, T., Tundel, R.E. & Buchwald, S.L. The selective reaction of aryl halides with KOH: synthesis of phenols, aromatic ethers, and benzofurans. J. Am. Chem. Soc. 128, 10694–10695 (2006).
Burgos, C.H., Barder, T.E., Huang, X. & Buchwald, S.L. Significantly improved method for the Pd-Catalyzed coupling of phenols with aryl halides: understanding ligand effects. Angew. Chem. Int. Ed. Engl. 45, 4321–4326 (2006).
Vorogushin, A.V., Huang, X. & Buchwald, S.L. Use of tunable ligands allows for intermolecular Pd-catalyzed C-O bond formation. J. Am. Chem. Soc. 127, 8146–8149 (2005).
Murata, M. & Buchwald, S.L. A general and efficient method for the palladium-catalyzed cross-coupling of thiols and secondary phosphines. Tetrahedron 60, 7397–7403 (2004).
Meyers, C. et al. Study of a new rate increasing 'base effect' in the Palladium-catalyzed amination of aryl iodides. J. Org. Chem. 69, 6010–6017 (2004).
Still, W.C., Kahn, M. & Mitra, A. A rapid chromatographic method. J. Org. Chem. 43, 2923–2925 (1978).
MacNeil, S.L., Wilson, B.J. & Snieckus, V. Anionic N-fries rearrangement of N-carbamoyl diarylamines to anthranilamides. Methodology and application to acridone and pyranoacridone alkaloids. Org. Lett. 8, 1133–1136 (2006).
Acknowledgements
Funding for this work was provided by the National Institutes of Health (GM 58160). We thank BASF, Saltigo and Chemetall for the gifts of Pd(OAc)2, XPhos and Cs2CO3, respectively. R.A.A. thanks Pfizer for a graduate fellowship. We also acknowledge Merck, Amgen and Boehringer Ingelheim for additional unrestricted fiscal support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The ligands and processes described in this protocol are the subjects of patents owned by MIT from which SLB receives royalties. These patents are licensed by Shasun Pharma Solutions (and their sublicensees), for whom SLB is a consultant and a member of the SAB, for the use of these ligands with this protocol.
Rights and permissions
About this article
Cite this article
Altman, R., Fors, B. & Buchwald, S. Pd-catalyzed amination reactions of aryl halides using bulky biarylmonophosphine ligands. Nat Protoc 2, 2881–2887 (2007). https://doi.org/10.1038/nprot.2007.414
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.414
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.