Abstract
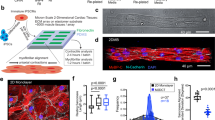
This protocol describes a method for attaching single isolated cardiac myocytes to carbon fibers for mechanical manipulation and measurement. This method relies on cell-adhesive carbon fibers that attach easily to the cell membrane without causing damage, and is thus applicable to intact myocytes. To connect the carbon fiber to micromanipulators, a fiber holder with glass capillaries must first be fabricated. After connection of the fibers to the micromanipulators, firm attachment is easily established by gently pressing the fiber tip onto the cell membrane. Unlike other methods, this technique does not require vast technical expertise, and therefore greatly facilitates experiments. This method enables detection of the effect of drugs, genetic defects or the expression of exogenous proteins on both active and passive properties of cardiac myocytes. In combination with other experimental procedures, this technique can also be applied to the study of mechano-transduction. This protocol can be completed in 3.5 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brady, A.J. Mechanical properties of isolated cardiac myocytes. Physiol. Rev. 71, 413–428 (1991).
Garnier, D. Attachment procedures for mechanical manipulation of isolated cardiac myocytes: a challenge. Cardiovasc. Res. 28, 1958–1964 (1994).
Tarr, M., Trank, J.W., Leiffer, P. & Shepherd, N. Sarcomere length-resting tension relation in single frog atrial cardiac cells. Circ. Res. 45, 554–559 (1979).
Tung, L. An ultrasensitive transducer for measurement of isometric contractile force from single heart cells. Pflugers Arch. 407, 109–115 (1986).
Fabiato, A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J. Gen. Physiol. 78, 457–497 (1981).
Brady, A.J., Tan, S.T. & Ricchiuti, N.V. Contractile force measured in unskinned isolated rat heart fibers. Nature 282, 728–729 (1979).
Palmer, R.E., Brady, A.J. & Roos, K.P. Mechanical measurement from isolated cardiac myocytes using a pipette attachment system. Am. J. Physiol. 270, C697–C704 (1996).
Hofmann, P.A. & Moss, R.L. Effects of calcium on shortening velocity in frog chemically skinned atrial myocytes and in mechanically disrupted ventricular myocardium from rat. Circ. Res. 70, 885–892 (1992).
Metzger, J.M. et al. Skeletal troponin C reduces contractile sensitivity to acidosis in cardiac myocytes from transgenic mice. Proc. Natl. Acad. Sci. USA 90, 9036–9040 (1993).
Bluhm, W.F., McCulloch, A.D. & Lew, W.Y. Active force in rabbit ventricular myocytes. J. Biomech. 28, 1119–1122 (1995).
Le Guennec, J.-Y., Peineau, N., Argibay, J.A., Mongo, K.G. & Garnier, D. A new method of attachment of isolated mammalian ventricular myocytes for tension recording: length dependence of passive and active tension. J. Mol. Cell. Cardiol. 22, 1083–1093 (1990).
Yasuda, S.I. et al. A novel method to study contraction characteristics of a single cardiac myocyte using carbon fibers. Am. J. Physiol. Heart Circ. Physiol. 281, H1442–H1446 (2001).
Nishimura, S. et al. Single cell mechanics of rat cardiomyocytes under isometric, unloaded and physiologically loaded conditions. Am. J. Physiol. 287, H196–H202 (2004).
Yasuda, S. et al. Unloaded shortening increases the peak of Ca2+ transients but accelerates their decay in rat single cardiac myocytes. Am. J. Physiol. 285, H470–H475 (2003).
Yasuda, S. et al. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature 463, 1025–1029 (2005).
Nishimura, S. et al. Contractile dysfunction of cardiomyopathic hamster myocytes is pronounced under high load conditions. J. Mol. Cell. Cardiol. 39, 231–239 (2005).
White, E., Boyett, M.R. & Orchard, C.H. The effects of mechanical loading and changes of length on single guinea-pig ventricular myocytes. J. Physiol. 482, 93–107 (1995).
Nishimura, S. et al. Expression of green fluorescent protein impairs the force-generating ability of isolated rat ventricular cardiomyocytes. Mol. Cell. Biochem. 286, 59–65 (2006).
Michele, D.E., Albayya, F.P. & Metzger, J.M. Direct, convergent hypersensitivity of calcium-activated force generation produced by hypertrophic cardiomyopathy mutant alpha-tropomyosins in adult cardiac myocytes. Nat. Med. 5, 1413–1417 (1999).
Yoneda, M. Force exerted by a single cilium of Mytilus edulus . I. Exp. Biol. 37, 461–468 (1960).
Nishimura, S. et al. Microtubule modulates the stiffness of cardiomyocytes against shear stress. Circ. Res. 98, 81–87 (2006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video
Length perturbation experiment in a single rat cardiac myocyte. The length of a cardiomyocyte was changed cyclically by applying a sinusoidal command of varying frequency (0.5 to 10 Hz) to the piezo-electric actuator connected to the carbon fiber. (MPG 3265 kb)
Rights and permissions
About this article
Cite this article
Sugiura, S., Nishimura, S., Yasuda, S. et al. Carbon fiber technique for the investigation of single-cell mechanics in intact cardiac myocytes. Nat Protoc 1, 1453–1457 (2006). https://doi.org/10.1038/nprot.2006.241
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.241
This article is cited by
-
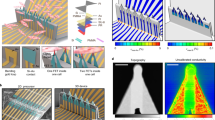
A guide for assessment of myocardial stiffness in health and disease
Nature Cardiovascular Research (2022)
-
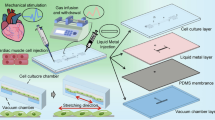
Microengineered platforms for characterizing the contractile function of in vitro cardiac models
Microsystems & Nanoengineering (2022)
-
Study of the union method of microelectrode array and AFM for the recording of electromechanical activities in living cardiomyocytes
European Biophysics Journal (2017)
-
Contractility assessment in enzymatically isolated cardiomyocytes
Biophysical Reviews (2012)
-
Micromechanical regulation in cardiac myocytes and fibroblasts: implications for tissue remodeling
Pflügers Archiv - European Journal of Physiology (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.