Abstract
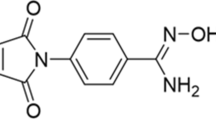
Peptides can be labeled with various trivalent radiometals for imaging or targeted radionuclide-therapy applications. The peptide is first conjugated to a chelating agent that is able to form stable complexes with the radionuclide of interest. This conjugation step can be carried out as part of the solid-phase peptide synthesis, or it can be undertaken in the solution phase after synthesis and purification of the peptide. The latter route, described here, involves reacting a molar excess of the activated tri-tert-butyl ester-derivatized chelator with a designated free amino group of a peptide analog, in which all other reactive amines are protected, in the presence of a coupling agent. The conjugate molecule is then purified prior to deprotection and further purification by HPLC. The product can be radiolabeled by addition of a suitable metal salt, followed, if necessary, by removal of the unchelated metal. The entire process of conjugation, purification and radiolabeling should take approximately 12.5 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Cooper, M.S., Sabbah, E. & Mather, S.J. Conjugation of chelating agents to proteins and radiolabelling with trivalent metallic isotopes. Nat. Protocols, published online 27 June 2006 (doi:10.1038/nprot.2006.49).
Achilefu, S. et al. Novel bioactive and stable neurotensin peptide analogues capable of delivering radiopharmaceuticals and molecular beacons to tumors. J. Med. Chem. 46, 3403–3411 (2003).
Chan, W.C. & White, P.D. (eds.) Fmoc Solid Phase Peptide Synthesis: A Practical Approach (Oxford University Press, Oxford, UK, 2000).
De Leon-Rodriguez, L.M., Kovacs, Z., Dieckmann, G.R. & Sherry, A.D. Solid-phase synthesis of DOTA-peptides. Chemistry 10, 1149–1155 (2004).
Reubi, J.C. et al. Unsulfated DTPA- and DOTA-CCK analogs as specific high-affinity ligands for CCK-B receptor-expressing human and rat tissues in vitro and in vivo. Eur. J. Nucl. Med. 25, 481–490 (1998).
Edreira, M., Melendez-Alafort, L. & Mather, S.J. Optimization of the small-scale synthesis of DOTA-Tyr3-octreotide. Nucl. Med. Commun. 23, 493–499 (2002).
Heppeler, A. et al. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour-targeting properties and potential for receptor-mediated internal radiotherapy. Chem. Eur. J. 5, 1974–1981 (1999).
Breeman, W.A. et al. Radiolabelling DOTA-peptides with 68Ga. Eur. J. Nucl. Med. Mol. Imaging 32, 478–485 (2005).
Breeman, W.A., De Jong, M., Visser, T.J., Erion, J.L. & Krenning, E.P. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur. J. Nucl. Med. Mol. Imaging 30, 917–920 (2003).
Hainsworth, J.E. & Mather, S.J. Regressive DOTA labelling performance with indium-111 and yttrium-90 over a week of use. Eur. J. Nucl. Med. Mol. Imaging 32, 1348 (2005).
Bio-Rad Laboratories. Chelex 100 and Chelex 20 Chelating Ion Exchange Resin Instruction Manual (Bio-Rad Laboratories, Hercules, CA, 2000).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sosabowski, J., Mather, S. Conjugation of DOTA-like chelating agents to peptides and radiolabeling with trivalent metallic isotopes. Nat Protoc 1, 972–976 (2006). https://doi.org/10.1038/nprot.2006.175
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.175
This article is cited by
-
Crown-hydroxylamines are pH-dependent chelating N,O-ligands with a potential for aerobic oxidation catalysis
Nature Communications (2023)
-
Design of PSMA ligands with modifications at the inhibitor part: an approach to reduce the salivary gland uptake of radiolabeled PSMA inhibitors?
EJNMMI Radiopharmacy and Chemistry (2021)
-
Preliminary in vitro comparison of 111In and 131I labeled nimotuzumabs
Journal of Radioanalytical and Nuclear Chemistry (2021)
-
Synthesis and Evaluation of Ga-68-Labeled Rhein for Early Assessment of Treatment-Induced Tumor Necrosis
Molecular Imaging and Biology (2020)
-
Evaluation of [131I]I- and [177Lu]Lu-DTPA-A11 Minibody for Radioimmunotherapy in a Preclinical Model of PSCA-Expressing Prostate Cancer
Molecular Imaging and Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.