Abstract
Radiopharmaceuticals play a vital role in cancer therapy. The carrier of radiopharmaceuticals can precisely locate and guide radionuclides to the target, where radionuclides kill surrounding tumor cells. Effective application of radiopharmaceuticals depends on the selection of an appropriate carrier. Herein, different types of carriers of radiopharmaceuticals and the characteristics are briefly described. Subsequently, we review radiolabeled monoclonal antibodies (mAbs) and their derivatives, and novel strategies of radiolabeled mAbs and their derivatives in the treatment of lymphoma and colorectal cancer. Furthermore, this review outlines radiolabeled peptides, and novel strategies of radiolabeled peptides in the treatment of neuroendocrine neoplasms, prostate cancer, and gliomas. The emphasis is given to heterodimers, bicyclic peptides, and peptide-modified nanoparticles. Last, the latest developments and applications of radiolabeled nucleic acids and small molecules in cancer therapy are discussed. Thus, this review will contribute to a better understanding of the carrier of radiopharmaceuticals and the application in cancer therapy.
Similar content being viewed by others
Facts
-
Radiopharmaceuticals hold great promise for the future of cancer therapy. Effective application of radiopharmaceuticals depends on the selection of an appropriate carrier.
-
The most employed carriers of radiopharmaceuticals include antibodies, peptides, nucleic acids, small molecules, and nanoparticles, each of which has advantages and disadvantages.
-
The high specificity and affinity of antibodies for target antigens make them an excellent carrier for radionuclide delivery. Antibody derivatives and the use of pretargeting strategies have further improved the therapeutic efficiency of radiolabeled antibodies.
-
Radiolabeled peptides are a very specific radiopharmaceutical group. There is a growing interest in the development of novel peptide carrier systems, such as heterodimers, bicyclic peptides, and peptide-modified nanoparticles.
Open questions
-
High specificity and affinity of the carrier for the target are crucial, and how can they achieve optimal?
-
Despite the increasing number of radiolabeled antibodies developed in preclinical studies, translation of the most prospective agents has remained challenging.
-
Radiolabeled peptides have undergone extensive preclinical studies and novel peptide carrier systems continue to be developed, but there are still challenges in their translation to clinical trials.
Introduction
Cancer is one of the leading causes of human death worldwide [1]. Despite increasing treatment options for cancer patients, such as radiotherapy, targeted therapy, and immunotherapy, etc., there is still need for treatment optimization [2]. Radiopharmaceutical therapy (RPT) as a novel therapeutic modality for cancer, attracted a high degree of recognition and commercial interest [3, 4]. RPT delivers radionuclides to tumor-related targets, where they can inhibit or destroy tumor tissues to achieve therapeutic purposes [5]. However, few radionuclides themselves can selectively target tumor sites, such as radioiodine targeted therapy for thyroid diseases [6], most radionuclides need to be guided by carriers to reach the target tissue [4, 7]. Therefore, the study of carrier systems related to radionuclide delivery has become an important area of a significant interest and growth. To date, a large number of studies have been carried out on the carrier of radiopharmaceuticals, and new carriers are constantly being explored and discovered [3, 8, 9]. Hence, in this review, we briefly describe the carrier of radiopharmaceuticals. Subsequently, radiolabeled antibodies are outlined, as well as novel approaches for the treatment of lymphoma and colorectal cancer (CRC) using radiolabeled antibodies. Furthermore, we review radiolabeled peptides, and novel strategies of radiolabeled peptides in the treatment of neuroendocrine neoplasms (NENs), prostate cancer, and gliomas, notably heterodimers, bicyclic peptides, and peptide-modified nanoparticles (NPs). Last, the latest developments and applications of radiolabeled nucleic acids and small molecules in cancer therapy are discussed. Thus, our elucidation will contribute to a better understanding of radiopharmaceutical carrier systems and the application in cancer therapy.
Carrier systems of radiopharmaceuticals
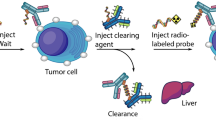
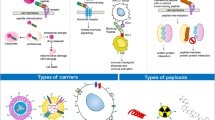
Radiopharmaceuticals generally consisting of radionuclides coupled to targeting carriers [10]. The carrier is responsible for selectively interacting with the target site, resulting in a higher concentration of radionuclides at the target site [11]. The specificity and selectivity required for pinpointing cancer cells for cell killing has been made possible by targeted carriers [4], as shown in Fig. 1a. Tumor cells express one or more proteins that are not expressed or are rarely expressed in normal tissues or other diseases, which can be precisely recognized and bound by carriers to deliver radionuclides to the tumor site. The ionizing radiation emitted by therapeutic radionuclides can cause damage to cellular DNA and other functional macromolecules, inhibiting or killing cancer cells while minimizing damage to healthy tissue [12, 13]. The selection of an ideal carrier to accurately deliver the radionuclide to specific tumor cells is the basis for achieving better therapeutic effect of RPT. The requirements of targeted carriers include high affinity and specificity for the target, non-toxic or non-immunogenic, and easy to produce and modify, etc. [11, 13, 14].
a Carriers of radiopharmaceuticals with high affinity and specificity for the tumor target can selectively guide therapeutic radionuclide payloads to tumor cells for targeted therapy. b The main categories of common carrier systems used for radiopharmaceutical development and advantages and disadvantages of various carriers.
Currently, the most employed carriers are antibodies, peptides, nucleic acids, small molecules, and NPs, etc., as shown in Fig. 1b. Antibodies were the first biological carriers employed for radiopharmaceuticals. The high affinity and specificity of antibodies for target antigens overexpressed on tumors make them excellent carriers for radionuclide delivery, especially monoclonal antibodies (mAbs) and their derivatives [15, 16]. However, intact mAbs have inherent main drawbacks due to their relatively high molecular weight, including slow pharmacokinetics and low diffusivity within solid tumors [17]. Although antibody fragments can improve the pharmacokinetics of solid tumor therapies, they are reduced in stability, show a significant degree of nonspecific accumulation in healthy tissues [17,18,19,20]. Peptides have many advantages that make them attractive radiopharmaceutical carriers, including ease of synthesis and radiolabeling, favorable pharmacokinetics, low toxicity and immunogenicity, etc. [21]. In particular, the development of heterodimeric peptides and bicyclic peptides provides powerful carrier systems [22, 23]. In recent years, nucleic acids are also being actively explored as carriers of radiopharmaceuticals, which have some significant advantages in terms of production, modification, possible targets, and immunogenicity, etc. [24]. Moreover, small molecule radiopharmaceuticals can rapidly penetrate tumors and be rapidly cleared from non-target tissues, etc., thus reducing toxicity, compared to large molecules [25]. This therapeutic approach is receiving increasing attention. Furthermore, the advanced hybrid radionuclide carriers are being developed, such as antibody- and peptide-modified NPs have been studied in radionuclide delivery and hold considerable promise in cancer therapy [26, 27]. This review focuses on radiolabeled antibodies, peptides, nucleic acids, small molecules and their novel strategies in cancer therapy.
Radiolabeled antibodies in cancer therapy
Approved radiolabeled mAbs and their derivatives
Currently, two radiolabeled anti-CD20 murine mAbs, 90Y-ibritumomab tiuxetan (Zevalin) and 131I-tositumomab (Bexxar) have been approved by the United States Food and Drug Administration (FDA) for the treatment of relapsed or refractory, low-grade or follicular B cell Non-Hodgkin lymphoma (NHL), including rituximab refractory follicular NHL in 2002 and 2003, respectively [28]. 131I-Metuximab for the treatment of hepatocellular carcinoma (HCC) and 131I-chTNT for the treatment of lung cancer have been approved by the China National Medical Products Administration [29, 30]. Clinical translation of radiolabeled antibodies therapy began with the approval of Zevalin and Bexxar. In a phase III study [31], the Zevalin RPT was compared with rituximab immunotherapy in 143 patients with relapsed or refractory low-grade, follicular, or transformed B‑NHL. Patients received a single intravenous dose of 0.4 mCI/kg of Zevalin (n = 73) or four weekly doses of 375 mg/m2 of rituximab (n = 70). The overall response rate for the Zevalin group and rituximab group were 80% and 56%, respectively, and complete response were 30% and 16%, respectively. Later, in a randomized phase III first-line indolent trial [32], Zevalin was shown to be highly valuable as first-line consolidation therapy in patients with advanced-stage follicular lymphoma. Among 409 patients available for analysis, estimated 8-year overall progression-free survival was 41% with Zevalin group versus 22% for control, and the median time to the next treatment was 8.1 years for Zevalin group versus 3.0 years in the control group. Additionally, in clinical trials of Bexxar, Kaminski et al. reported very encouraging results in patients with follicular lymphoma [33]. In this study, 76 patients with stage III or IV follicular lymphoma receiving Bexxar as initial treatment. Results showed that overall response rate was as high as 95%, and 75% of patients having a complete response. After a median follow-up of 5.1 years, 5-year progression-free survival was 59% and median progression-free survival was 6.1 years. These advances then opened the door widely to cancer therapy.
Novel strategies of radiolabeled mAbs and their derivatives in cancer therapy
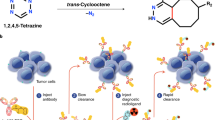
Non-Hodgkin lymphoma
After the first approval, a significant number of preclinical and clinical studies evaluated the efficacy of radiolabeled antibodies in lymphomas and therapeutic strategies, particularly anti-CD20 RPT (Table 1 and Fig. 2a) [34, 35]. Radiolabeled anti-CD20 chimeric mAb rituximab has large potential to be employed for NHL. Rituximab has the ability to induce antibody-dependent cellular cytotoxicity and apoptosis via intrinsic and extrinsic apoptotic pathways [36, 37]. A phase II study demonstrated that 99% of NHL patients showed a complete or partial response to 131I-labeled rituximab, and the treatment had low toxicity and was less expensive than chemotherapy [38]. Additionally, other novel strategies to improve the efficiency of RPT are also being developed. Pretargeted RPT is a novel promising approach aiming to improve clearance and reduce off-target toxicity, therefore minimizing toxicity and maximizing therapeutic response [39], including the streptavidin-biotin and bispecific antibodies (bsAbs) pretargeting approaches. In streptavidin-biotin method, mAbs and radioactive agents are used separately (Fig. 2a). Biotin has a higher affinity to streptavidin than the average affinity of antigen-antibody, and a streptavidin molecule can bind to multiple radioactive biotins. Based on this, higher doses of radiation can be delivered to the targeted tissue [39]. Preclinical studies have shown that streptavidin-biotin RPT has better efficacy and lower toxicity than directly radiolabeled mAbs in lymphoma xenografts [40,41,42]. However, streptavidin-biotin RPT is limited by its immunogenicity and interference with endogenous biotin, which may complicate the clinical translation of this approach [43]. BsAbs pretargeting approach involves the use of unlabeled bsAbs with affinity to both radiolabeled hapten and tumor-associated antigen (Fig. 2a). According to the bsAbs pretargeting approach, one arm of bsAb targets the tumor antigen and the other arm recognizes the radiolabeled hapten used for RPT [44]. This pretargeting approach improves the therapeutic response of targeted radionuclides in NHL compared to directly radiolabeled mAbs [45]. For example, Sharkey et al. reported that pretargeting method using anti-CD20 bsAb TF4 followed by 90Y-DOTA-peptide significantly improved survival, and cured 33% to 90% of lymphoma nude mice, even at relatively low dose [46]. Based on these studies, it can be concluded that pretargeting is a promising cancer therapy approach that can improve the efficiency of RPT. Other effective strategies are also being explored.
a The strategy of targeting CD20 for the treatment of NHL and some corresponding radiopharmaceuticals. Major therapeutic strategies include direct radiolabeled antibodies, streptavidin-biotin pretargeting, and bsAb pretargeting approaches. b The strategy of targeting CEA for the treatment of colorectal cancer and some corresponding radiopharmaceuticals. Major therapeutic strategies include direct radiolabeled antibodies and antibody fragments, and bsAb pretargeting approaches.
Colorectal cancer
Radiolabeled antibodies for the treatment of CRC have been investigated in various preclinical and clinical trials (Table 1). The most commonly targeted antigens include carcinoembryonic antigen (CEA), epithelial cell-adhesion molecule, and the colon specific antigen p, etc., as shown in Fig. 2b [47]. CEA is expressed in ~95% of CRC and is the most commonly used targeted antigen [48]. To date, a number of clinical trials have evaluated the efficacy of anti-CEA RPT (Table 1) (Fig. 2b). In 2017, a phase II study of 131I-labeled anti-CEA mAb labetuzumab in CRC showed that the median time to progression and overall survival for all 63 patients was 16 months and 55 months, respectively [49]. In addition, bsAb pretargeting approach has been developed for CEA targeting in CRC (Fig. 2b). For example, preclinical and clinical studies have demonstrated that pretargeting with bsAb (TF2) and radiolabeled hapten peptide (IMP288) specifically and rapidly targeted tumors, and inhibited tumor growth [50, 51]. Furthermore, radiolabeled anti-CEA antibody fragments, such as minibody [52], F(ab’)2 [53], and diabody [54] have also been used in therapeutic studies of CRC (Fig. 2b). Wong et al. reported that 123I-labeled anti-CEA cT84.66 minibody showed tumor targeting to CRC and had a faster clearance compared to intact antibodies [52]. Thus, radiolabeled antibodies have shown promising potential in CRC.
Radiolabeled peptides in cancer therapy
Approved radiolabeled peptides
Radiolabeled somatostatin (SST) analogs, 177Lu-DOTA-TATE was approved by the FDA for the treatment of adult patients with SSTR-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in 2018 [55]. SST is a cyclic disulfide-bond-containing peptide hormones that can bind to five somatostatin receptor subtypes (SSTR1-5) widely expressed in the whole body [56, 57]. Indeed, structure-function studies have shown that the small tetrapeptide Phe-Trp-Lys-Thr is necessary for biological activity (Fig. 3a) [58]. Recently, in the final analysis of overall survival and long-term safety of the phase III midgut NETs trial (NCT01578239) [59], the median overall survival of the 177Lu-DOTA-TATE group was 48.0 months and the control group was 36.3 months, which did not reach statistical significance. However, the 11.7-month difference in median overall survival with 177Lu-DOTA-TATE might be considered clinically relevant, and no new safety signals were reported at the time of the final analysis. Interestingly, Wu et al. showed that in a human xenograft tumor model of NET, 177Lu-DOTATATE RPT led to increased infiltration of CD86+ antigen presenting cells and CD49b+/FasL+ NK cells in tumor tissues [60]. Therefore, further investigation of the immunomodulatory role of RPT will be essential to improve the efficacy of cancer therapy. Undoubtedly, the clinical success of radiolabeled SST analogs has paved the way for novel peptide derivatives.
a Development of radiolabeled somatostatin analogs for the treatment of neuroendocrine neoplasms. b Radiolabeled somatostatin receptor agonists and antagonists for neuroendocrine neoplasm therapy. c Comparison of radiolabeled somatostatin receptor agonists and antagonists in neuroendocrine neoplasm therapy.
Novel strategies of radiolabeled peptides in cancer therapy
Neuroendocrine neoplasms
NENs overexpress specific peptide receptors, particularly somatostatin receptors (SSTRs) (Fig. 3b) [61]. Radiolabeled somatostatin (SST) analogs targeting SSTRs are the most advanced radiopharmaceuticals for NENs (Table 2 and Fig. 3) [62, 63]. There are two major categories of SST analogs: agonists and antagonists (Fig. 3b) [63]. The agonist octreotide was the first synthesized SST analog, and was the starting point for the development of radiolabeled SST analogs (Fig. 3a) [64]. The two prevalent radiolabeled structures DOTATOC and DOTATATE are also agonists (Fig. 3b) [65]. In 2020, Ballal et al. reported 225Ac-labeled DOTATATE for 32 patients with GEP-NETs, which showed partial remission in 15 patients and stable disease in 9 [66]. In addition, the phase III trial evaluating the efficacy and safety of 177Lu-DOTA-TOC in patients with GEP-NETs is ongoing (NCT03049189), with estimated completion by June 2029. Furthermore, the introduction of SSTRs antagonists, such as LM3, and JR11, is an important advance in the field of SSTRs targeting [63]. Preclinical and clinical studies have shown that SSTR antagonists have better binding ability to SSTR, a higher tumor uptake rate, and can deliver a higher dose of radiation than agonists (Fig. 3c) [67,68,69]. For example, the antagonist 177Lu-DOTA-JR11 had longer tumor retention time and higher tumor uptake compared to 177Lu-DOTATATE in patients with NETs [68]. Obviously, the use of radiolabeled SSTR antagonists may offer more successful therapy strategies for NETs compared to agonists and warrant further validation.
Prostate cancer
Prostate cancer overexpresses specific receptors, including gastrin-releasing peptide receptor (GRPR), prostate specific membrane antigen (PSMA), and integrins, etc. (Fig. 4a) [70]. Radiolabeled bombesin (BBN) analogs exhibit high binding affinity and specificity to GRPR, and its research in the field of GRPR-positive cancer therapy has been thriving [71]. The first radiolabeled BNN analog for therapeutic application is the potent GRPR agonist 177Lu-AMBA, which binds to GRPR with high affinity, showing high therapeutic potential [72, 73]. However, a phase I escalation study in patients with metastatic castration-resistant prostate cancer (mCRPC) was discontinued due to severe adverse effects after injection of therapeutic doses of 177Lu-AMBA [74]. In addition, multiple studies have shown that radiolabeled GRPR antagonists are better than agonists for cancer therapy [75]. Therefore, preclinical and clinical studies have shifted from GRPR agonists to antagonists. For example, GRPR antagonist 67/68Ga/111In/177Lu-NeoBOMB1 exhibited high GRPR binding affinity, high tumor uptake efficiency, and high in vivo metabolic stability in preclinical studies [76]. A phase I/II study is underway to evaluate 177Lu-NeoBOMB1 (NCT03872778), and is expected to be completed in October 2025. Furthermore, in recent years, researchers have been developing radiolabeled heterodimers (Fig. 4a). For example, heterodimeric peptides (RGD-Glu-[90Y-DO3A]-6-Ahx-RM2) and peptides linked to small molecules (125I-BO530) [56, 77]. Compared with the corresponding monovalent peptide ligands, heterodimers can simultaneously or independently bind to different target receptors, resulting in stronger binding to target cells [22]. In 2019, Abouzayed et al. synthesized the GRPR/PSMA-targeting bispecific heterodimer BO530 [56], a small molecule linked to the BBN-based antagonist RM26 (Fig. 4b). It was shown that 125I-labeled BO530 specifically bound to both GRPR and PSMA in vitro with high affinity and cellular retention. Similarly, it was specific to both targets in vivo, has long activity retention in tumors and was cleared from normal organs. Moreover, radiolabeled peptide-modified NPs have been used to study prostate cancer therapy (Fig. 4a) [57]. For example, Wang et al. reported that modification of multifunctional porous silicon NPs (PSi NPs) with iRGD peptide enhanced the uptake and retention of NPs in mice bearing prostate cancer xenografts [27]. Thus, radiolabeled peptides have ushered in a novel prospect for prostate cancer therapy.
Gliomas
Glioma cells express specific receptors and glycoproteins on their surface, such as neurokinin type 1 receptor (NK1R), integrins, and MMP-14, etc. (Fig. 5a) [58]. Substance P is the natural ligand of NK1R, thus radiolabeled substance P analogs can be used for targeting NK1R to treat gliomas (Table 2) [78]. In the phase I study of 90Y-DOTAGA-SP in glioblastoma multiforme [79], all patients were well tolerated without acute toxicity. In 2019, 213Bi-labeled DOTA-SP was examined in patients with recurrent glioblastoma [80], the median overall survival of patients was 23.6 months, and the median overall survival after recurrence was 10.9 months compares favorably to standard therapy. Additionally, radiolabeled heterodimeric peptides have shown extremely favorable results in preclinical studies for gliomas. For example, in 2022, Liu et al. used radiolabeled heterodimeric peptides iRGD-C6-lys(211At-ATE)-C6-DA7R targeting both integrins and VEGF receptor for glioma therapy [81], which significantly inhibited tumor growth and prolonged the survival of tumor-bearing mice. Furthermore, radiolabeled bicyclic peptides hold tremendous promise in cancer therapy, and are currently under development. Bicyclic peptides can be synthetically manufactured and bind targets with high affinity and selectivity, providing high tumor penetration and rapid excretion from normal tissue [23]. In 2019, Eder et al. reported radiolabeled bicyclic peptides with subnanometer affinity for MMP-14, which showed selective tumor uptake in a mouse model [23]. Moreover, nanomedicine-based approaches are being used to develop innovative treatment strategies for glioblastoma (Fig. 5) [82,83,84]. In 2022, Silva et al. designed 177Lu-labeled AuNPs carrying substance P derivatives [82], which showed significant radiobiological effects in glioblastoma cells with high cellular uptake and internalization, reduced cell viability and survival, deserving further preclinical evaluation (Fig. 5b). In conclusion, these studies bring hope for new radiolabeled peptide in cancer therapy.
Radiolabeled nucleic acids in cancer therapy
Nucleic acid-based carriers consist of various structural variants. Antisense oligonucleotides (ASO) and nucleic acid aptamers as radiopharmaceutical carriers are very promising (Fig. 6) [24, 85]. Currently, radiolabeled ASOs have achieved a series of successes. In 2016, Kang et al. successfully prepared 99mTc radiolabeled anti-miRNA-155 (AMO-155) oligonucleotide and tested it in vivo in multiple tumor models, demonstrating its high stability and effective delivery [86]. Additionally, more efficient strategies are being developed to improve ASO delivery (Fig. 6a). Firstly, using cell-penetrating peptide-based nanoprobes. For example, in 2021, Yang et al. constructed a novel noncovalent antisense nanoprobe, 99mTc-labeled AMOs/cell-penetrating peptide (CPPs) PepFect6 [87], which showed higher cellular uptake and retention, had low cytotoxicity, high specificity, and sensitivity. Secondly, optimizing the labeling method. In 2022, Chen et al. developed a new tetrapeptide for direct labeling AMO-21 with 99mTc [88]. This labeling approach has high labeling efficiency and stable labeling. Thirdly, the application of nucleic acid analogs antisense peptide nucleic acid (PNA). For example, in 2020, Jiang et al. developed a 99mTc-labeled PNA probe targeting miR-155, which had superior specificity and targeting ability in vitro and in vivo [89]. Fourthly, using PNA-mediated pretargeting. Preclinical studies have shown that this pretargeting is a promising approach for cancer therapy [90, 91]. For example, in 2021, Tano et al. evaluated novel 177Lu-labeled PNA probes for the pretargeting (Fig. 6b) [92]. They designed second-generation hybridization probes, the anti-HER2 affibody-PNA primary probe and the complementary secondary probes, and labeled with 177Lu. It was shown that the primary agent bound with high specificity and affinity to HER2-expressing cells in vitro, and the secondary hybridization probes that bound with high affinity to the primary agent. In vivo studies showed HER2-specific uptake of all 177Lu radiolabeled probes in xenografts in pretargeting. In 2022, Oroujeni et al. reported that the combination of PNA-mediated pretargeting and trastuzumab significantly increased survival in mice bearing HER2-expressing xenografts [93]. Finally, using oligonucleotide functionalized NPs. For example, in 2021, Bavelaar et al. first reported 111In-labeled oligonucleotide-functionalized AuNP constructs, which markedly improved the uptake of oligonucleotides and possessed telomerase-specific antiproliferative and cytotoxic effects [94]. In 2022, Ren et al. evaluated the liposome encapsulated 99mTc-labeled ASO probe, which showed good stability and targeting ability [95]. Aptamers are also a highly potential radiopharmaceutical carrier, consisting of functional RNA or single-stranded DNA that can generate unique and diverse tertiary structure to interact with their targets with high affinity and specificity (Fig. 6c) [96]. In this regard, Li et al. prepared the 64Cu-labeled oligonucleotide aptamer AS1411 binding to surface nucleolin, which showed reasonable in vivo stability and high binding affinity to cells [97]. Furthermore, aptamer functionalized NPs are also of interest in the delivery of radionuclides [84, 98] (Fig. 6c, d). For example, Bandekar et al. evaluated the therapeutic effect of A10 PSMA aptamer-labeled liposomes loaded with 225Ac, which selectively bind, become internalized, and kill PSMA-expressing cells [98]. This may have potential application value in cancer therapy.
Radiolabeled small molecules in cancer therapy
Radiolabeled small molecules for cancer therapy are rapidly evolving, particularly targeting PSMA for prostate cancer therapy [99]. In 2022, radiolabeled small molecule 177Lu-PSMA-617 was approved by the FDA for the treatment of PSMA-positive mCRPC after androgen receptor pathway inhibition and taxane-based chemotherapy (Fig. 7a) [100]. Additionally, it was reported that radiolabeled heterodimers improved the specificity and accuracy of prostate cancer therapy (Fig. 7b) [56, 101]. For example, Abouzayed et al. synthesized PSMA/GRPR-targeting heterodimer 125I-BO530 formed by linking PSMA-617 and peptide, which exhibited targeting specificity and long activity retention both in vitro and in vivo [56]. Small molecule-modified NPs also enhanced the specificity of NPs targeting cancer cells (Fig. 7b). In 2022, Cheng et al. reported that the inclusion of the highly specific PSMA-targeting ligand enabled 111In/177Lu-nanotexaphyrin to preferential accumulation in PSMA-positive prostate tumors and successfully inhibited tumor growth in a xenograft model [102]. Furthermore, multiple other preclinical and clinical studies are also evaluating novel strategies targeting PSMA for prostate cancer therapy, including monotherapy and combination therapy (Fig. 7c). Many studies of monotherapy are underway. Two phase III trials of 177Lu-PSMA-I&T for mCRPC underway to evaluate its safety and efficacy in mCRPC patients (NCT04647526) (NCT05204927). However, a significant number of patients (up to 30%) do not respond or develop resistance to monotherapy with β-emitter 177Lu-labeled PSMA-targeted therapy [103, 104], so there is interest in using other radionuclides, notably α-emitter 225Ac. A phase I study evaluating the safety of 225Ac-PSMA-617 in prostate cancer patients is currently ongoing, with estimated completion by July 2025 (NCT04597411). At the same time, a phase II trial of 225Ac-PSMA-I&T for mCRPC is underway, which is expected to be completed in December 2024 (NCT05219500). Several trials are investigating PSMA-RPT in combination with other treatment modalities to improve cancer therapy, including chemotherapy (NCT05340374), immunotherapy [105], and targeted therapies (NCT03874884) [106]. For example, Czernin et al. found that PSMA RPT and PD-1 blockade have synergistic anti-tumor effects [105]. Combination of 225Ac-PSMA-617 and anti-PD-1 dramatically improved disease control in a mouse model of prostate cancer compared with either monotherapy. Survival was extended to 51.5 d (control, 28 d; anti-PD-1, 37 d; 225Ac-PSMA-617, 32 d; anti-PD-1, 37 d). Besides, RPT targeting PSMA themselves have been combined. For example, co-administration of 177Lu-PSMA-617 and 225Ac-PSMA-617 [107]. Interestingly, there is a phase I/II clinical trial combining 177Lu-PSMA-I&T and the mAb 225Ac-J591 for progressive mCRPC, and the estimated study completion date is December 2027 (NCT04886986). Small molecule-based RPT has made significant progress in the past few years, and this therapeutic approach is likely to play an increasingly significant role in the coming years.
Radiolabeled nanoparticles in cancer therapy
The use of NPs as radiopharmaceutical carriers is developing rapidly, which further improves the therapeutic efficacy of radiopharmaceuticals. NPs can be broadly classified into organic and inorganic NPs (Fig. 1b) [9, 108]. Organic NPs including liposomes, dendrimers, and polymeric NPs. Inorganic NPs including gold NPs, silica NPs, and carbon-based NPs. They have been widely discussed as radionuclide carriers in cancer therapy [9, 109]. In 2022, Huang et al. demonstrated that intravenous injection of 211At-labeled AuNPs 211At-AuNPs@mPEG significantly inhibited tumor growth in a pancreatic cancer model [110], providing a new framework for the design of NPs suitable for RPT via intravenous injection. In addition, NPs have been effectively functionalized with peptides, antibodies, and aptamers, etc. for specific binding to tumor receptors, and the number of these targeting ligands bound to a NP can be much more than one [111, 112]. For example, González-Ruíz et al. evaluated the therapeutic effect of the aptamer and the peptide-labeled AuNPs loaded with 177Lu, which significantly decreased the tumor cell viability in vitro and in vivo, and inhibited tumor progression [84]. In recent years, many preclinical studies have been conducted of radiolabeled NPs and they are expected to play a critical role in cancer therapy.
Conclusion and perspective
Currently, RPT is emerging as a safe and effective approach to treat many types of cancer. The efficacy of RPT depends on a variety of factors, including the properties of the tumor (permeability, degree of vascularization, and blood flow), the properties of the target (specificity, density, and heterogeneity of expression), the properties of the radionuclide, and the targeting carrier [113]. Therapeutic radionuclides can be reliably attached to carriers and targeted delivery to almost all types of tumors owing to the developed techniques and protocols [114]. We provide an overview of the most commonly employed radiopharmaceutical carrier systems in cancer therapy. Each of these carriers reviewed has advantages and disadvantages, and their characteristics may even change depending on the modifiers or radionuclides used. Therefore, the translation of most carriers into clinics has not progressed as quickly as expected.
Antibody-based radiopharmaceuticals have been widely employed in cancer therapy. However, only two were approved by the FDA. The application of radiolabeled antibodies in solid cancers remains a challenge [3]. It is interesting to note that new discoveries about antibody engineering or radionuclides, and the use of pretargeting strategies are trying to overcome these problems. Radiolabeled peptide is a relatively new and very specific radiopharmaceutical group. There is a growing interest in the development of stable and well-defined novel peptide carrier systems, such as heterodimers and cyclic peptides, which bring hope for new radiolabeled peptides in cancer therapy [23, 81]. However, the available evidence in this regard is limited and further research is needed to fully evaluate its potential. Currently, the development and application of nucleic acids are still in their infancy, and although oligonucleotide-based radiopharmaceuticals have achieved a series of successes in the biomedical field, clinical translation remains slow and many challenges remain to be addressed. Encouragingly, radiolabeled small molecules have made significant progress in mCRPC. 177Lu-PSMA-617 has been approved by the FDA, and multiple other clinical trials using different radionuclides and conjugates are underway. Furthermore, antibodies, peptides, nucleic acids, and small molecule-modified NPs are of great interest due to the extraordinary advantages they provided, and are currently being actively investigated. Currently, the scientific community has shown increased interest in developing advanced hybrid radionuclide carriers [84]. Advancement in the field of RPT may further be enabled by using different carrier systems with higher radionuclide loads. However, the safe and effective delivery of radiopharmaceuticals to tumor tissues is still a great challenge in cancer therapy. Thus, there still exists much work to be done in this field in the future, more effective carrier systems remain to be explored and developed. At the same time, combined with chemotherapy, external beam radiotherapy, molecular targeted therapy, and other therapies, RPT has the potential to reach its full therapeutic efficacy in tumor treatment. In short, the field related to radionuclide delivery is still a challenging frontier, with many promising carrier systems waiting to be explored.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Zhang Y, Lu L, Song F, Zou X, Liu Y, Zheng X, et al. Research progress on non-protein-targeted drugs for cancer therapy. J Exp Clin cancer Res. 2023;42:62.
Rondon A, Rouanet J, Degoul F. Radioimmunotherapy in oncology: overview of the last decade clinical trials. Cancers. 2021;13:5570.
Sgouros G, Bodei L, McDevitt MR, Nedrow JR. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020;19:589–608.
St James S, Bednarz B, Benedict S, Buchsbaum JC, Dewaraja Y, Frey E, et al. Current status of radiopharmaceutical therapy. Int J Radiat Oncol Biol Phys. 2021;109:891–901.
Zheng L, Zhang L, Tang L, Huang D, Pan D, Guo W, et al. Gut microbiota is associated with response to (131)I therapy in patients with papillary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2023;50:1453–65.
Peltek OO, Muslimov AR, Zyuzin MV, Timin AS. Current outlook on radionuclide delivery systems: from design consideration to translation into clinics. J Nanobiotechnol. 2019;17:90.
Krecisz P, Czarnecka K, Krolicki L, Mikiciuk-Olasik E, Szymanski P. Radiolabeled peptides and antibodies in medicine. Bioconjug Chem. 2021;32:25–42.
Pellico J, Gawne PJ. Radiolabelling of nanomaterials for medical imaging and therapy. Chem Soc Rev. 2021;50:3355–423.
Vermeulen K, Vandamme M, Bormans G, Cleeren F. Design and challenges of radiopharmaceuticals. Semin Nucl Med. 2019;49:339–56.
Kleynhans J, Grobler AF, Ebenhan T, Sathekge MM, Zeevaart JR. Radiopharmaceutical enhancement by drug delivery systems: a review. J Control Release. 2018;287:177–93.
Aerts A, Impens NR, Gijs M, D’Huyvetter M, Vanmarcke H, Ponsard B, et al. Biological carrier molecules of radiopharmaceuticals for molecular cancer imaging and targeted cancer therapy. Curr Pharm Des. 2014;20:5218–44.
Gudkov SV, Shilyagina NY, Vodeneev VA, Zvyagin AV. Targeted radionuclide therapy of human tumors. Int J Mol Sci. 2015;17:33.
Dash A, Knapp FF, Pillai MR. Targeted radionuclide therapy—an overview. Curr Radiopharm. 2013;6:152–80.
Parakh S, Lee ST, Gan HK, Scott AM. Radiolabeled antibodies for cancer imaging and therapy. Cancers. 2022;14:1454.
Xenaki KT, Oliveira S, van Bergen En Henegouwen PMP. Antibody or antibody fragments: implications for molecular imaging and targeted therapy of solid tumors. Front Immunol. 2017;8:1287.
Sharma R, Suman SK, Mukherjee A. Antibody-based radiopharmaceuticals as theranostic agents: an overview. Curr Med Chem. 2022;29:5979–6005.
White JM, Escorcia FE, Viola NT. Perspectives on metals-based radioimmunotherapy (RIT): moving forward. Theranostics. 2021;11:6293–314.
Fu R, Carroll L, Yahioglu G, Aboagye EO, Miller PW. Antibody fragment and affibody immunoPET imaging agents: radiolabelling strategies and applications. ChemMedChem. 2018;13:2466–78.
Kraeber-Bodere F, Rousseau C, Bodet-Milin C, Frampas E, Faivre-Chauvet A, Rauscher A, et al. A pretargeting system for tumor PET imaging and radioimmunotherapy. Front Pharm. 2015;6:54.
Mohtavinejad N, Shafiee Ardestani M, Khalaj A, Pormohammad A, Najafi R, Bitarafan-Rajabi A, et al. Application of radiolabeled peptides in tumor imaging and therapy. Life Sci. 2020;258:118206.
Cheng X, Hubner R, von Kiedrowski V, Fricker G, Schirrmacher R, Wangler C, et al. Design, synthesis, in vitro and in vivo evaluation of heterobivalent SiFAlin-modified peptidic radioligands targeting both integrin alpha(v)beta(3) and the MC1 receptor-suitable for the specific visualization of melanomas? Pharmaceuticals. 2021;14:547.
Eder M, Pavan S, Bauder-Wüst U, van Rietschoten K, Baranski AC, Harrison H, et al. Bicyclic peptides as a new modality for imaging and targeting of proteins overexpressed by tumors. Cancer Res. 2019;79:841–52.
Winkler J. Oligonucleotide conjugates for therapeutic applications. Ther Deliv. 2013;4:791–809.
Wester HJ, Schottelius M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin Nucl Med. 2019;49:302–12.
Dziawer L, Majkowska-Pilip A, Gawel D, Godlewska M, Pruszynski M, Jastrzebski J, et al. Trastuzumab-modified gold nanoparticles labeled with (211)At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials. 2019;9:632.
Wang CF, Sarparanta MP, Mäkilä EM, Hyvönen ML, Laakkonen PM, Salonen JJ, et al. Multifunctional porous silicon nanoparticles for cancer theranostics. Biomaterials. 2015;48:108–18.
Sachpekidis C, Jackson DB, Soldatos TG. Radioimmunotherapy in non-Hodgkin’s lymphoma: retrospective adverse event profiling of Zevalin and Bexxar. Pharmaceuticals. 2019;12:141.
Tang M, Li WL, Li JY, Lv J, Chen FK, Zhu JL, et al. Analysis of factors influencing the distribution of 131-I in combined treatment of Licartin with transcatheter arterial chemoembolization in primary hepatic carcinoma. Front Oncol. 2022;12:993948.
Zhao Z, Su Z, Zhang W, Luo M, Wang H, Huang L. A randomized study comparing the effectiveness of microwave ablation radioimmunotherapy and postoperative adjuvant chemoradiation in the treatment of non-small cell lung cancer. J BUON. 2016;21:326–32.
Witzig TE, Gordon LI, Cabanillas F, Czuczman MS, Emmanouilides C, Joyce R, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453–63.
Morschhauser F, Radford J, Van Hoof A, Botto B, Rohatiner AZ, Salles G, et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the International, Randomized, Phase III First-LineIndolent trial. J Clin Oncol. 2013;31:1977–83.
Kaminski MS, Tuck M, Estes J, Kolstad A, Ross CW, Zasadny K, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med. 2005;352:441–9.
Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–74.
Cicone F, Santo G, Bodet-Milin C, Cascini GL, Kraeber-Bodere F, Stokke C, et al. Radioimmunotherapy of non-Hodgkin B-cell lymphoma: an update. Semin Nucl Med. 2023;53:413–25.
Flieger D, Renoth S, Beier I, Sauerbruch T, Schmidt-Wolf I. Mechanism of cytotoxicity induced by chimeric mouse human monoclonal antibody IDEC-C2B8 in CD20-expressing lymphoma cell lines. Cell Immunol. 2000;204:55–63.
Jazirehi AR, Bonavida B. Cellular and molecular signal transduction pathways modulated by rituximab (rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: implications in chemosensitization and therapeutic intervention. Oncogene. 2005;24:2121–43.
McQuillan AD, Macdonald WB, Turner JH. Phase II study of first-line (131)I-rituximab radioimmunotherapy in follicular non-Hodgkin lymphoma and prognostic (18)F-fluorodeoxyglucose positron emission tomography. Leuk Lymphoma. 2015;56:1271–7.
Eskian M, Khorasanizadeh M, Zinzani PL, Illidge TM, Rezaei N. Novel methods to improve the efficiency of radioimmunotherapy for non-Hodgkin lymphoma. Int Rev Immunol. 2019;38:79–91.
Press OW, Corcoran M, Subbiah K, Hamlin DK, Wilbur DS, Johnson T, et al. A comparative evaluation of conventional and pretargeted radioimmunotherapy of CD20-expressing lymphoma xenografts. Blood. 2001;98:2535–43.
Green DJ, Pagel JM, Pantelias A, Hedin N, Lin Y, Wilbur DS, et al. Pretargeted radioimmunotherapy for B-cell lymphomas. Clin Cancer Res. 2007;13:5598s–603s.
Park SI, Shenoi J, Pagel JM, Hamlin DK, Wilbur DS, Orgun N, et al. Conventional and pretargeted radioimmunotherapy using bismuth-213 to target and treat non-Hodgkin lymphomas expressing CD20: a preclinical model toward optimal consolidation therapy to eradicate minimal residual disease. Blood. 2010;116:4231–9.
Weiden PL, Breitz HB, Press O, Appelbaum JW, Bryan JK, Gaffigan S, et al. Pretargeted radioimmunotherapy (PRIT) for treatment of non-Hodgkin’s lymphoma (NHL): initial phase I/II study results. Cancer Biother Radiopharm. 2000;15:15–29.
Altai M, Membreno R, Cook B, Tolmachev V, Zeglis BM. Pretargeted imaging and therapy. J Nucl Med. 2017;58:1553–9.
Sharkey RM, Karacay H, Chang CH, McBride WJ, Horak ID, Goldenberg DM. Improved therapy of non-Hodgkin’s lymphoma xenografts using radionuclides pretargeted with a new anti-CD20 bispecific antibody. Leukemia. 2005;19:1064–9.
Sharkey RM, Karacay H, Litwin S, Rossi EA, McBride WJ, Chang CH, et al. Improved therapeutic results by pretargeted radioimmunotherapy of non-Hodgkin’s lymphoma with a new recombinant, trivalent, anti-CD20, bispecific antibody. Cancer Res. 2008;68:5282–90.
Koppe MJ, Bleichrodt RP, Oyen WJ, Boerman OC. Radioimmunotherapy and colorectal cancer. Br J Surg. 2005;92:264–76.
Koppe MJ, Postema EJ, Aarts F, Oyen WJ, Bleichrodt RP, Boerman OC. Antibody-guided radiation therapy of cancer. Cancer Metastasis Rev. 2005;24:539–67.
Sahlmann CO, Homayounfar K, Niessner M, Dyczkowski J, Conradi LC, Braulke F, et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer. 2017;123:638–49.
Schoffelen R, Boerman OC, Goldenberg DM, Sharkey RM, van Herpen CM, Franssen GM, et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: first clinical results. Br J Cancer. 2013;109:934–42.
Heskamp S, Hernandez R, Molkenboer-Kuenen JDM, Essler M, Bruchertseifer F, Morgenstern A, et al. alpha- versus beta-emitting radionuclides for pretargeted radioimmunotherapy of carcinoembryonic antigen-expressing human colon cancer xenografts. J Nucl Med. 2017;58:926–33.
Wong JY, Chu DZ, Williams LE, Yamauchi DM, Ikle DN, Kwok CS, et al. Pilot trial evaluating an 123I-labeled 80-kilodalton engineered anticarcinoembryonic antigen antibody fragment (cT84.66 minibody) in patients with colorectal cancer. Clin Cancer Res. 2004;10:5014–21.
Ychou M, Azria D, Menkarios C, Faurous P, Quenet F, Saint-Aubert B, et al. Adjuvant radioimmunotherapy trial with iodine-131-labeled anti-carcinoembryonic antigen monoclonal antibody F6 F(ab’)2 after resection of liver metastases from colorectal cancer. Clin Cancer Res. 2008;14:3487–93.
Olafsen T, Cheung CW, Yazaki PJ, Li L, Sundaresan G, Gambhir SS, et al. Covalent disulfide-linked anti-CEA diabody allows site-specific conjugation and radiolabeling for tumor targeting applications. Protein Eng Des Sel. 2004;17:21–7.
Hennrich U, Kopka K. Lutathera((R)): the first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals. 2019;12:114.
Abouzayed A, Yim CB, Mitran B, Rinne SS, Tolmachev V, Larhed M, et al. Synthesis and preclinical evaluation of radio-iodinated GRPR/PSMA bispecific heterodimers for the theranostics application in prostate cancer. Pharmaceutics. 2019;11:358.
Lankoff A, Czerwinska M, Kruszewski M. Nanoparticle-based radioconjugates for targeted imaging and therapy of prostate cancer. Molecules. 2023;28:4122.
Bolcaen J, Kleynhans J, Nair S, Verhoeven J, Goethals I, Sathekge M, et al. A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma. Theranostics. 2021;11:7911–47.
Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, et al. (177)Lu-Dotatate plus long-acting octreotide versus high‑dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:1752–63.
Wu Y, Pfeifer AK, Myschetzky R, Garbyal RS, Rasmussen P, Knigge U, et al. Induction of anti-tumor immune responses by peptide receptor radionuclide therapy with (177)Lu-DOTATATE in a murine model of a human neuroendocrine tumor. Diagnostics. 2013;3:344–55.
Refardt J, Hofland J, Kwadwo A, Nicolas GP, Rottenburger C, Fani M, et al. Theranostics in neuroendocrine tumors: an overview of current approaches and future challenges. Rev Endocr Metab Disord. 2021;22:581–94.
Fani M, Mansi R, Nicolas GP, Wild D. Radiolabeled somatostatin analogs-a continuously evolving class of radiopharmaceuticals. Cancers. 2022;14:1172.
Eychenne R, Bouvry C, Bourgeois M, Loyer P, Benoist E, Lepareur N. Overview of radiolabeled somatostatin analogs for cancer imaging and therapy. Molecules. 2020;25:4012.
Del Olmo-Garcia MI, Prado-Wohlwend S, Andres A, Soriano JM, Bello P, Merino-Torres JF. Somatostatin and somatostatin receptors: from signaling to clinical applications in neuroendocrine neoplasms. Biomedicines. 2021;9:1810.
Shi M, Jakobsson V, Greifenstein L, Khong PL, Chen X, Baum RP, et al. Alpha-peptide receptor radionuclide therapy using actinium-225 labeled somatostatin receptor agonists and antagonists. Front Med. 2022;9:1034315.
Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with (225)Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to (177)Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47:934–46.
Nicolas GP, Mansi R, McDougall L, Kaufmann J, Bouterfa H, Wild D, et al. Biodistribution, pharmacokinetics, and dosimetry of (177)Lu-, (90)Y-, and (111)In-labeled somatostatin receptor antagonist OPS201 in comparison to the agonist (177)Lu-DOTATATE: the mass effect. J Nucl Med. 2017;58:1435–41.
Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55:1248–52.
Dalm SU, Nonnekens J, Doeswijk GN, de Blois E, van Gent DC, Konijnenberg MW, et al. Comparison of the therapeutic response to treatment with a 177Lu-labeled somatostatin receptor agonist and antagonist in preclinical models. J Nucl Med. 2016;57:260–5.
Stott Reynolds TJ, Smith CJ, Lewis MR. Peptide-based radiopharmaceuticals for molecular imaging of prostate cancer. Adv Exp Med Biol. 2018;1096:135–58.
Mansi R, Nock BA, Dalm SU, Busstra MB, van Weerden WM, Maina T. Radiolabeled bombesin analogs. Cancers. 2021;13:5766.
Lantry LE, Cappelletti E, Maddalena ME, Fox JS, Feng W, Chen J, et al. 177Lu-AMBA: synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. J Nucl Med. 2006;47:1144–52.
Maddalena ME, Fox J, Chen J, Feng W, Cagnolini A, Linder KE, et al. 177Lu-AMBA biodistribution, radiotherapeutic efficacy, imaging, and autoradiography in prostate cancer models with low GRP-R expression. J Nucl Med. 2009;50:2017–24.
Bodei L, Ferrari M, Nunn AD. 177Lu-AMBA Bombesin analogue in hormone refractory prostate cancer patients: a phase I escalation study with single-cycle administrations. 2007.
Lymperis E, Kaloudi A, Kanellopoulos P, Krenning EP, de Jong M, Maina T, et al. Comparative evaluation of the new GRPR-antagonist (111) In-SB9 and (111) In-AMBA in prostate cancer models: Implications of in vivo stability. J Label Comp Radiopharm. 2019;62:646–55.
Nock BA, Kaloudi A, Lymperis E, Giarika A, Kulkarni HR, Klette I, et al. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist NeoBOMB1: preclinical and first clinical results. J Nucl Med. 2017;58:75–80.
Bandara N, Stott Reynolds TJ, Schehr R, Bandari RP, Diebolder PJ, Krieger S, et al. Matched-pair, (86)Y/(90)Y-labeled, bivalent RGD/bombesin antagonist, [RGD-Glu-[DO3A]-6-Ahx-RM2], as a potential theranostic agent for prostate cancer. Nucl Med Biol. 2018;62–63:71–7.
Kneifel S, Cordier D, Good S, Ionescu MC, Ghaffari A, Hofer S, et al. Local targeting of malignant gliomas by the diffusible peptidic vector 1,4,7,10-tetraazacyclododecane-1-glutaric acid-4,7,10-triacetic acid-substance p. Clin Cancer Res. 2006;12:3843–50.
Cordier D, Forrer F, Kneifel S, Sailer M, Mariani L, Macke H, et al. Neoadjuvant targeting of glioblastoma multiforme with radiolabeled DOTAGA-substance P—results from a phase I study. J Neurooncol. 2010;100:129–36.
Krolicki L, Bruchertseifer F, Kunikowska J, Koziara H, Krolicki B, Jakucinski M, et al. Safety and efficacy of targeted alpha therapy with (213)Bi-DOTA-substance P in recurrent glioblastoma. Eur J Nucl Med Mol Imaging. 2019;46:614–22.
Liu W, Ma H, Liang R, Chen X, Li H, Lan T, et al. Targeted alpha therapy of glioma using (211)At-labeled heterodimeric peptide targeting both VEGFR and integrins. Mol Pharmaceutics. 2022;19:3206–16.
Silva F, D’Onofrio A, Mendes C, Pinto C, Marques A, Campello MPC, et al. Radiolabeled gold nanoseeds decorated with substance P peptides: synthesis, characterization and in vitro evaluation in glioblastoma cellular models. Int J Mol Sci. 2022;23:617.
Arora G, Dubey P, Shukla J, Ghosh S, Bandopadhyaya G. Evaluation of cytotoxic and tumor targeting capability of (177)Lu-DOTATATE-nanoparticles: a trailblazing strategy in peptide receptor radionuclide therapy. Ann Nucl Med. 2016;30:334–45.
González-Ruíz A, Ferro-Flores G, Jiménez-Mancilla N, Escudero-Castellanos A, Ocampo-García B, Luna-Gutiérrez M, et al. In vitro and in vivo synergistic effect of radiotherapy and plasmonic photothermal therapy on the viability of cancer cells using 177Lu–Au-NLS-RGD-Aptamer nanoparticles under laser irradiation. J Radioanal Nucl Chem. 2018;318:1913–21.
Chaturvedi S, Mishra AK. Vectors for the delivery of radiopharmaceuticals in cancer therapeutics. Ther Deliv. 2014;5:893–912.
Kang L, Huo Y, Ji Q, Fan S, Yan P, Zhang C, et al. Noninvasive visualization of microRNA-155 in multiple kinds of tumors using a radiolabeled anti-miRNA oligonucleotide. Nucl Med Biol. 2016;43:171–8.
Yang G, Zhao Y, Gong A, Miao W, Yan L, Nie P, et al. Improved cellular delivery of antisense oligonucleotide for miRNA-21 imaging in vivo using cell-penetrating peptide-based nanoprobes. Mol Pharm. 2021;18:787–95.
Chen Z, Yang Q, Song L, Qiu Y, Wu S, Huang W, et al. A novel tetrapeptide for chelator-free radiolabeling in optimized preparation of (99m)Tc-radiolabeled oligonucleotides. Am J Nucl Med Mol imaging. 2022;12:143–51.
Jiang Y, Gai Y, Long Y, Liu Q, Liu C, Zhang Y, et al. Application and evaluation of [(99m)Tc]-labeled peptide nucleic acid targeting microRNA-155 in breast cancer imaging. Mol Imaging. 2020;19:1536012120916124.
Myrhammar A, Vorobyeva A, Westerlund K, Yoneoka S, Orlova A, Tsukahara T, et al. Evaluation of an antibody-PNA conjugate as a clearing agent for antibody-based PNA-mediated radionuclide pretargeting. Sci Rep. 2020;10:20777.
Westerlund K, Altai M, Mitran B, Konijnenberg M, Oroujeni M, Atterby C, et al. Radionuclide therapy of HER2-expressing human xenografts using affibody-based peptide nucleic acid-mediated pretargeting: in vivo proof of principle. J Nucl Med. 2018;59:1092–8.
Tano H, Oroujeni M, Vorobyeva A, Westerlund K, Liu Y, Xu T, et al. Comparative evaluation of novel (177)Lu-labeled PNA probes for affibody-mediated PNA-based pretargeting. Cancers. 2021;13:500.
Oroujeni M, Tano H, Vorobyeva A, Liu Y, Vorontsova O, Xu T, et al. Affibody-mediated PNA-based pretargeted cotreatment improves survival of trastuzumab-treated mice bearing HER2-expressing xenografts. J Nucl Med. 2022;63:1046–51.
Bavelaar BM, Song L, Jackson MR, Able S, Tietz O, Skaripa-Koukelli I, et al. Oligonucleotide-functionalized gold nanoparticles for synchronous telomerase inhibition, radiosensitization, and delivery of theranostic radionuclides. Mol Pharmaceutics. 2021;18:3820–31.
Ren J, Zhang X, Cao J, Tian J, Luo J, Yu Y, et al. Radiosynthesis of a novel antisense imaging probe targeting LncRNA HOTAIR in malignant glioma. BMC Cancer. 2022;22:79.
Kim DH, Seo JM, Shin KJ, Yang SG. Design and clinical developments of aptamer-drug conjugates for targeted cancer therapy. Biomater Res. 2021;25:42.
Li J, Zheng H, Bates PJ, Malik T, Li XF, Trent JO, et al. Aptamer imaging with Cu-64 labeled AS1411: preliminary assessment in lung cancer. Nucl Med Biol. 2014;41:179–85.
Bandekar A, Zhu C, Jindal R, Bruchertseifer F, Morgenstern A, Sofou S. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular alpha-particle therapy of cancer. J Nucl Med. 2014;55:107–14.
Cheng L, Yang T, Zhang J, Gao F, Yang L, Tao W. The application of radiolabeled targeted molecular probes for the diagnosis and treatment of prostate cancer. Korean J Radio. 2023;24:574–89.
Fallah J, Agrawal S, Gittleman H, Fiero MH, Subramaniam S, John C, et al. FDA approval summary: lutetium Lu 177 vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2023;29:1651–7.
Boinapally S, Lisok A, Lofland G, Minn I, Yan Y, Jiang Z, et al. Hetero-bivalent agents targeting FAP and PSMA. Eur J Nucl Med Mol Imaging. 2022;49:4369–81.
Cheng MHY, Overchuk M, Rajora MA, Lou JWH, Chen Y, Pomper MG, et al. Targeted theranostic (111)In/Lu-nanotexaphyrin for SPECT imaging and photodynamic therapy. Mol Pharmaceutics. 2021;19:1803–13.
Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Rahbar K, Bode A, Weckesser M, Avramovic N, Claesener M, Stegger L, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41:522–8.
Czernin J, Current K, Mona CE, Nyiranshuti L, Hikmat F, Radu CG, et al. Immune-checkpoint blockade enhances (225)Ac-PSMA617 efficacy in a mouse model of prostate cancer. J Nucl Med. 2021;62:228–31.
Gafita A, Marcus C, Kostos L, Schuster DM, Calais J, Hofman MS. Predictors and real-world use of prostate-specific radioligand therapy: PSMA and beyond. Am Soc Clin Oncol Educ Book. 2022;42:1–17.
Langbein T, Kulkarni HR, Schuchardt C, Mueller D, Volk GF, Baum RP. Salivary gland toxicity of PSMA-targeted radioligand therapy with (177)Lu-PSMA and combined (225)Ac- and (177)Lu-labeled PSMA ligands (TANDEM-PRLT) in advanced prostate cancer: a single-center systematic investigation. Diagnostics. 2022;12:1926.
Aghebati-Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235:1962–72.
Kamkaew A, Ehlerding EB, Cai W. Nanoparticles as radiopharmaceutical vectors. In: Lewis JS, Windhorst AD, Zeglis BM (eds). Radiopharmaceutical Chemistry. (Springer Nature Switzerland AG, Switzerland, 2019) 181–203.
Huang X, Kaneda-Nakashima K, Kadonaga Y, Kabayama K, Shimoyama A, Ooe K, et al. Astatine-211-labeled gold nanoparticles for targeted alpha-particle therapy via intravenous injection. Pharmaceutics. 2022;14:2705.
Hernandez-Jimenez T, Cruz-Nova P, Ancira-Cortez A, Gibbens-Bandala B, Lara-Almazan N, Ocampo-Garcia B, et al. Toxicity assessment of [(177)Lu]Lu-iFAP/iPSMA nanoparticles prepared under GMP-compliant radiopharmaceutical processes. Nanomaterials. 2022;12:4181.
Murar M, Pujals S, Albertazzi L. Multivalent effect of peptide functionalized polymeric nanoparticles towards selective prostate cancer targeting. Nanoscale Adv. 2023;5:1378–85.
Knox SJ, Meredith RF. Clinical radioimmunotherapy. Semin Radiat Oncol. 2000;10:73–93.
Salih S, Alkatheeri A, Alomaim W, Elliyanti A. Radiopharmaceutical treatments for cancer therapy, radionuclides characteristics, applications, and challenges. Molecules. 2022;27:5231.
Forrer F, Oechslin-Oberholzer C, Campana B, Herrmann R, Maecke HR, Mueller-Brand J, et al. Radioimmunotherapy with 177Lu-DOTA-rituximab: final results of a phase I/II Study in 31 patients with relapsing follicular, mantle cell, and other indolent B-cell lymphomas. J Nucl Med. 2013;54:1045–52.
Thakral P, Singla S, Vashist A, Yadav MP, Gupta SK, Tyagi JS, et al. Preliminary experience with yttrium-90-labelled rituximab (chimeric anti CD-20 antibody) in patients with relapsed and refractory B cell non-Hodgkins lymphoma. Curr Radiopharm. 2016;9:160–8.
Durand-Panteix S, Monteil J, Sage M, Garot A, Clavel M, Saidi A, et al. Preclinical study of (212)Pb alpha-radioimmunotherapy targeting CD20 in non-Hodgkin lymphoma. Br J Cancer. 2021;125:1657–65.
Havlena GT, Kapadia NS, Huang P, Song H, Engles J, Brechbiel M, et al. Cure of micrometastatic B-cell lymphoma in a SCID mouse model using (213)bi-anti-CD20 monoclonal antibody. J Nucl Med. 2023;64:109–16.
Kang L, Li C, Rosenkrans ZT, Huo N, Chen Z, Ehlerding EB, et al. CD38-targeted theranostics of lymphoma with (89)Zr/(177)Lu-labeled daratumumab. Adv Sci. 2021;8:2001879.
Vilhelmsson Timmermand O, Larsson E, Ulmert D, Tran TA, Strand S. Radioimmunotherapy of prostate cancer targeting human kallikrein-related peptidase 2. EJNMMI Res. 2016;6:27.
Li J, Huang T, Hua J, Wang Q, Su Y, Chen P, et al. CD46 targeted (212)Pb alpha particle radioimmunotherapy for prostate cancer treatment. J Exp Clin Cancer Res. 2023;42:61.
Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–91.
Mohajershojai T, Jha P, Bostrom A, Frejd FY, Yazaki PJ, Nestor M. In vitro characterization of (177)Lu-DOTA-M5A anti-carcinoembryonic antigen humanized antibody and HSP90 inhibition for potentiated radioimmunotherapy of colorectal cancer. Front Oncol. 2022;12:849338.
Zhang L, Zhao S, Jiang H, Zhang R, Zhang M, Pan W, et al. Radioimmunotherapy study of (131)I-labeled Atezolizumab in preclinical models of colorectal cancer. EJNMMI Res. 2022;12:70.
Li HK, Morokoshi Y, Kodaira S, Kusumoto T, Minegishi K, Kanda H, et al. Utility of (211)At-trastuzumab for the treatment of metastatic gastric cancer in the liver: evaluation of a preclinical alpha-radioimmunotherapy approach in a clinically relevant mouse model. J Nucl Med. 2021;62:1468–74.
Meredith RF, Khazaeli MB, Plott WE, Spencer SA, Wheeler RH, Brady LW, et al. Initial clinical evaluation of iodine-125-labeled chimeric 17-1A for metastatic colon cancer. J Nucl Med. 1995;36:2229–33.
Divgi CR, Scott AM, Dantis L, Capitelli P, Siler K, Hilton S, et al. Phase I radioimmunotherapy trial with iodine-131-CC49 in metastatic colon carcinoma. J Nucl Med. 1995;36:586–92.
Sharkey RM, Goldenberg DM, Vagg R, Pawlyk D, Wong GY, Siegel JA, et al. Phase I clinical evaluation of a new murine monoclonal antibody (Mu-9) against colon-specific antigen-p for targeting gastrointestinal carcinomas. Cancer. 1994;73:864–77.
Timmermand OV, Elgqvist J, Beattie KA, Orbom A, Larsson E, Eriksson SE, et al. Preclinical efficacy of hK2 targeted [(177)Lu]hu11B6 for prostate cancer theranostics. Theranostics. 2019;9:2129–42.
Casey JL, Napier MP, King DJ, Pedley RB, Chaplin LC, Weir N, et al. Tumour targeting of humanised cross-linked divalent-Fab’ antibody fragments: a clinical phase I/II study. Br J Cancer. 2002;86:1401–10.
Bellaye PS, Moreau M, Raguin O, Oudot A, Bernhard C, Vrigneaud JM, et al. Radiolabeled F(ab’)(2)-cetuximab for theranostic purposes in colorectal and skin tumor-bearing mice models. Clin Transl Oncol. 2018;20:1557–70.
Weber T, Botticher B, Arndt MA, Mier W, Sauter M, Exner E, et al. Preclinical evaluation of a diabody-based (177)Lu-radioimmunoconjugate for CD22-directed radioimmunotherapy in a non-Hodgkin lymphoma mouse model. Cancer Lett. 2016;381:296–304.
Tsai WK, Zettlitz KA, Dahlbom M, Reiter RE, Wu AM. Evaluation of [(131)I]I- and [(177)Lu]Lu-DTPA-A11 minibody for radioimmunotherapy in a preclinical model of PSCA-expressing prostate cancer. Mol Imaging Biol. 2020;22:1380–91.
D’Huyvetter M, Vincke C, Xavier C, Aerts A, Impens N, Baatout S, et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics. 2014;4:708–20.
Puttemans J, Dekempeneer Y, Eersels JL, Hanssens H, Debie P, Keyaerts M, et al. Preclinical targeted alpha- and beta(-)-radionuclide therapy in HER2-positive brain metastasis using camelid single-domain antibodies. Cancers. 2020;12:1017.
Kraeber-Bodéré F, Faivre-Chauvet A, Ferrer L, Vuillez JP, Brard PY, Rousseau C, et al. Pharmacokinetics and dosimetry studies for optimization of anti-carcinoembryonic antigen x anti-hapten bispecific antibody-mediated pretargeting of Iodine-131-labeled hapten in a phase I radioimmunotherapy trial. Clin Cancer Res. 2003;9:3973s–81s.
Gonzalez GP, Garcia IG, Gonzalez JG, Sanchez LP, Mirabal MV, Marin CC, et al. Phase I clinical trial of the (131)I-labeled anticarcinoembryonic antigen CIGB-M3 multivalent antibody fragment. Cancer Biother Radiopharm. 2011;26:353–63.
Liu Y, Vorobyeva A, Orlova A, Konijnenberg MW, Xu T, Bragina O, et al. Experimental therapy of HER2-expressing xenografts using the second-generation HER2-targeting affibody molecule (188)Re-ZHER2:41071. Pharmaceutics. 2022;14:1092.
Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. (2)(1)(3)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41:2106–19.
Behnammanesh H, Jokar S, Erfani M, Geramifar P, Sabzevari O, Amini M, et al. Design, preparation and biological evaluation of a (177)Lu-labeled somatostatin receptor antagonist for targeted therapy of neuroendocrine tumors. Bioorg Chem. 2020;94:103381.
Baum RP, Zhang J, Schuchardt C, Muller D, Macke H. First-in-humans study of the SSTR antagonist (177)Lu-DOTA-LM3 for peptide receptor radionuclide therapy in patients with metastatic neuroendocrine neoplasms: dosimetry, safety, and efficacy. J Nucl Med. 2021;62:1571–81.
Cullinane C, Jeffery CM, Roselt PD, van Dam EM, Jackson S, Kuan K, et al. Peptide receptor radionuclide therapy with (67)Cu-CuSarTATE is highly efficacious against a somatostatin-positive neuroendocrine tumor model. J Nucl Med. 2020;61:1800–5.
Kurth J, Krause BJ, Schwarzenbock SM, Bergner C, Hakenberg OW, Heuschkel M. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [(177)Lu]Lu-RM2: a radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:123–35.
Lymperis E, Kaloudi A, Sallegger W, Bakker IL, Krenning EP, de Jong M, et al. Radiometal-dependent biological profile of the radiolabeled gastrin-releasing peptide receptor antagonist SB3 in cancer theranostics: metabolic and biodistribution patterns defined by neprilysin. Bioconjug Chem. 2018;29:1774–84.
Escudero-Castellanos A, Ocampo-García B, Ferro-Flores G, Santos-Cuevas C, Morales-Ávila E, Luna-Gutiérrez M, et al. Synthesis and preclinical evaluation of the 177Lu-DOTA-PSMA(inhibitor)-Lys3-bombesin heterodimer designed as a radiotheranostic probe for prostate cancer. Nucl Med Commun. 2019;40:278–86.
Zoghi M, Attar Nosrati S, Rogni F, Shirvani G, Johari Daha F. Preclinical evaluation of new GnRH-I receptor radionuclide therapy with (177) Lu-peptide tracer. J Label Comp Radiopharm. 2019;62:310–20.
Krolicki L, Bruchertseifer F, Kunikowska J, Koziara H, Pawlak D, Kulinski R, et al. Dose escalation study of targeted alpha therapy with [(225)Ac]Ac-DOTA-substance P in recurrence glioblastoma—safety and efficacy. Eur J Nucl Med Mol Imaging. 2021;48:3595–605.
Majkowska-Pilip A, Koźmiński P, Wawrzynowska A, Budlewski T, Kostkiewicz B, Gniazdowska E. Application of neurokinin-1 receptor in targeted strategies for glioma treatment. Part I: synthesis and evaluation of substance P fragments labeled with (99m)Tc and (177)Lu as potential receptor radiopharmaceuticals. Molecules. 2018;23:2542.
Vilchis-Juarez A, Ferro-Flores G, Santos-Cuevas C, Morales-Avila E, Ocampo-Garcia B, Diaz-Nieto L, et al. Molecular targeting radiotherapy with cyclo-RGDFK(C) peptides conjugated to 177Lu-labeled gold nanoparticles in tumor-bearing mice. J Biomed Nanotechnol. 2014;10:393–404.
Klingler M, Summer D, Rangger C, Haubner R, Foster J, Sosabowski J, et al. DOTA-MGS5, a new cholecystokinin-2 receptor-targeting peptide analog with an optimized targeting profile for theranostic use. J Nucl Med. 2019;60:1010–6.
Qin Y, Imobersteg S, Blanc A, Frank S, Schibli R, Béhé MP, et al. Evaluation of actinium-225 labeled minigastrin analogue [(225)Ac]Ac-DOTA-PP-F11N for targeted alpha particle therapy. Pharmaceutics. 2020;12:1088.
Herrmann K, Schottelius M, Lapa C, Osl T, Poschenrieder A, Hanscheid H, et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu- and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J Nucl Med. 2016;57:248–51.
Osl T, Schmidt A, Schwaiger M, Schottelius M, Wester HJ. A new class of PentixaFor- and PentixaTher-based theranostic agents with enhanced CXCR4-targeting efficiency. Theranostics. 2020;10:8264–80.
Leonte RA, Chilug LE, Serban R, Mustaciosu C, Raicu A, Manda G, et al. Preparation and preliminary evaluation of neurotensin radiolabelled with (68)Ga and (177)Lu as potential theranostic agent for colon cancer. Pharmaceutics. 2021;13:506.
Persson M, Rasmussen P, Madsen J, Ploug M, Kjaer A. New peptide receptor radionuclide therapy of invasive cancer cells: in vivo studies using 177Lu-DOTA-AE105 targeting uPAR in human colorectal cancer xenografts. Nucl Med Biol. 2012;39:962–9.
Zhang X, Chen F, Turker MZ, Ma K, Zanzonico P, Gallazzi F, et al. Targeted melanoma radiotherapy using ultrasmall (177)Lu-labeled alpha-melanocyte stimulating hormone-functionalized core-shell silica nanoparticles. Biomaterials. 2020;241:119858.
Velikyan I, Bulenga TN, Selvaraju R, Lubberink M, Espes D, Rosenström U, et al. Dosimetry of [(177)Lu]-DO3A-VS-Cys(40)-Exendin-4-impact on the feasibility of insulinoma internal radiotherapy. Am J Nucl Med Mol Imaging. 2015;5:109–26.
Acknowledgements
This work was supported by grants from the National Key R&D project of the Chinese Ministry of Science and Technology [2018YFE0205100]; the Key Program of the National Natural Science Foundation of China [11875299]; Chengguan District Science and Technology Bureau Project [2022SHFZQ022]; Innovation Group Project of Basic Research in Gansu Province [23JRRA569].
Author information
Authors and Affiliations
Contributions
Study concept and design: HZ, QL and CD. Drafting of the manuscript: TZ. Critical revision of the manuscript for important intellectual content: HL, XC, ZD, BY, WS, WW, XJ, TK and BW. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, T., Lei, H., Chen, X. et al. Carrier systems of radiopharmaceuticals and the application in cancer therapy. Cell Death Discov. 10, 16 (2024). https://doi.org/10.1038/s41420-023-01778-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-023-01778-3