Abstract
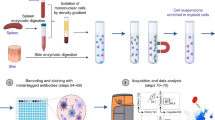
Dendritic cells (DCs) are central to the induction of immune responses and are a pivotal control point that determines the outcome of infectious challenge. Cannulation of afferent lymphatic vessels allows the isolation of large numbers of lymph DCs. First, lymph nodes that are draining the skin are surgically removed (takes approximately 1 h). Over a period of 6–8 weeks, afferent lymphatic vessels re-anastomose with the efferent duct, forming larger 'pseudoafferent' lymphatic vessels that can be surgically cannulated. Surgical cannulation takes 2 h to perform; daily maintenance of the catheter requires 30 min. Isolation of lymph cells requires 1 h and an additional 60–180 min to enrich or purify the DCs. The lymph can be harvested for up to 1 month, with relatively constant cell numbers and subset distribution throughout this period. This technique, although technically demanding, facilitates studies of DCs and other cells that traffic in the lymph in both the steady state and following antigenic exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hein, W.R., McClure, S.J. & Miyasaka, M. Cellular composition of peripheral lymph and skin of sheep defined by monoclonal antibodies. Int. Arch. Allergy Appl. Immunol. 84, 241–246 (1987).
Howard, C.J., Sopp, P., Brownlie, J., Parsons, K.R. & Lee, L.S. Phenotypic variation and functional differences within dendritic cells isolated from afferent lymph. Adv. Exp. Med. Biol. 378, 105–107 (1995).
Liu, L.M. & MacPherson, G.G. Antigen processing: cultured lymph-borne dendritic cells can process and present native protein antigens. Immunology 84, 241–246 (1995).
Huang, F.P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000).
Steinman, R.M. & Nussenzweig, M.C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA 99, 351–358 (2002).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Emery, D.L., MacHugh, N.D. & Ellis, J.A. The properties and functional activity of non-lymphoid cells from bovine afferent (peripheral) lymph. Immunology 62, 177–183 (1987).
Schwartz-Cornil, I. et al. Probing leukocyte traffic in lymph from oro-nasal mucosae by cervical catheterization in a sheep model. J. Immunol. Methods 305, 152–161 (2005).
Hanrahan, C.F. et al. Cellular requirements for the activation and proliferation of ruminant gammadelta T cells. J. Immunol. 159, 4287–4294 (1997).
Yawalkar, N., Hunger, R.E., Pichler, W.J., Braathen, L.R. & Brand, C.U. Human afferent lymph from normal skin contains an increased number of mainly memory / effector CD4(+) T cells expressing activation, adhesion and co-stimulatory molecules. Eur. J. Immunol. 30, 491–497 (2000).
Bonneau, M. et al. Migratory monocytes and granulocytes are major lymphatic carriers of Salmonella from tissue to draining lymph node. J. Leukoc. Biol. 79, 268–276 (2006).
McKeever, D.J., MacHugh, N.D., Goddeeris, B.M., Awino, E. & Morrison, W.I. Bovine afferent lymph veiled cells differ from blood monocytes in phenotype and accessory function. J. Immunol. 147, 3703–3709 (1991).
Miller, H.R. & Adams, E.P. Reassortment of lymphocytes in lymph from normal and allografted sheep. Am. J. Pathol. 87, 59–80 (1977).
Haig, D.M., Hopkins, J. & Miller, H.R. Local immune responses in afferent and efferent lymph. Immunology 96, 155–163 (1999).
Howard, C.J. et al. Identification of two distinct populations of dendritic cells in afferent lymph that vary in their ability to stimulate T cells. J. Immunol. 159, 5372–5382 (1997).
Bujdoso, R., Hopkins, J., Dutia, B.M., Young, P. & McConnell, I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J. Exp. Med. 170, 1285–1301 (1989).
Hopkins, J., Dutia, B.M., Bujdoso, R. & McConnell, I. In vivo modulation of CD1 and MHC class II expression by sheep afferent lymph dendritic cells. Comparison of primary and secondary immune responses. J. Exp. Med. 170, 1303–1318 (1989).
Epardaud, M. et al. Enrichment for a CD26hi SIRP-subset in lymph dendritic cells from the upper aero-digestive tract. J. Leukoc. Biol. 76, 553–561 (2004).
Hein, W.R., Barber, T., Cole, S.A., Morrison, L. & Pernthaner, A. Long-term collection and characterization of afferent lymph from the ovine small intestine. J. Immunol. Methods 293, 153–168 (2004).
Liu, L., Zhang, M., Jenkins, C. & MacPherson, G.G. Dendritic cell heterogeneity in vivo: two functionally different dendritic cell populations in rat intestinal lymph can be distinguished by CD4 expression. J. Immunol. 161, 1146–1155 (1998).
Liu, L.M. & MacPherson, G.G. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J. Exp. Med. 177, 1299–1307 (1993).
Liu, L.M. & MacPherson, G.G. Rat intestinal dendritic cells: immunostimulatory potency and phenotypic characterization. Immunology 85, 88–93 (1995).
Yrlid, U. & Macpherson, G. Phenotype and function of rat dendritic cell subsets. Apmis. 111, 756–765 (2003).
Bimczok, D., Sowa, E.N., Faber-Zuschratter, H., Pabst, R. & Rothkotter, H.J. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur. J. Immunol. 35, 1418–1427 (2005).
Gliddon, D.R. & Howard, C.J. CD26 is expressed on a restricted subpopulation of dendritic cells in vivo. Eur. J. Immunol. 32, 1472–1481 (2002).
Howard, C.J. & Hope, J.C. Dendritic cells, implications on function from studies of the afferent lymph veiled cell. Vet. Immunol. Immunopathol. 77, 1–13 (2000).
Belz, G.T., Heath, W.R. & Carbone, F.R. The role of dendritic cell subsets in selection between tolerance and immunity. Immunol. Cell Biol. 80, 463–468 (2002).
Despars, G. & O'Neill, H.C. A role for niches in the development of a multiplicity of dendritic cell subsets. Exp. Hematol. 32, 235–243 (2004).
Iwasaki, A. & Kelsall, B.L. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190, 229–239 (1999).
Gliddon, D.R., Hope, J.C., Brooke, G.P. & Howard, C.J. DEC-205 expression on migrating dendritic cells in afferent lymph. Immunology 111, 262–272 (2004).
Acknowledgements
The authors thank members of the bovine immunology groups at the Institute of Animal Health, Compton, UK. We gratefully acknowledge the staff of the animal facilities for care of the cattle. The work was funded by the Biotechnology and Biological Sciences Research Council, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hope, J., Howard, C., Prentice, H. et al. Isolation and purification of afferent lymph dendritic cells that drain the skin of cattle. Nat Protoc 1, 982–987 (2006). https://doi.org/10.1038/nprot.2006.125
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.125
This article is cited by
-
Collection of lymph-borne dendritic cells in the rat
Nature Protocols (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.