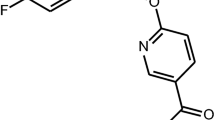
Abstract
Blonanserin differs from currently used serotonin 5-HT2A/dopamine-D2 receptor antagonists in that it exhibits higher affinity for dopamine-D2/3 receptors than for serotonin 5-HT2A receptors. We investigated the involvement of dopamine-D3 receptors in the effects of blonanserin on cognitive impairment in an animal model of schizophrenia. We also sought to elucidate the molecular mechanism underlying this involvement. Blonanserin, as well as olanzapine, significantly ameliorated phencyclidine (PCP)-induced impairment of visual-recognition memory, as demonstrated by the novel-object recognition test (NORT) and increased extracellular dopamine levels in the medial prefrontal cortex (mPFC). With blonanserin, both of these effects were antagonized by DOI (a serotonin 5-HT2A receptor agonist) and 7-OH-DPAT (a dopamine-D3 receptor agonist), whereas the effects of olanzapine were antagonized by DOI but not by 7-OH-DPAT. The ameliorating effect was also antagonized by SCH23390 (a dopamine-D1 receptor antagonist) and H-89 (a protein kinase A (PKA) inhibitor). Blonanserin significantly remediated the decrease in phosphorylation levels of PKA at Thr197 and of NR1 (an essential subunit of N-methyl-D-aspartate (NMDA) receptors) at Ser897 by PKA in the mPFC after a NORT training session in the PCP-administered mice. There were no differences in the levels of NR1 phosphorylated at Ser896 by PKC in any group. These results suggest that the ameliorating effect of blonanserin on PCP-induced cognitive impairment is associated with indirect functional stimulation of the dopamine-D1-PKA-NMDA receptor pathway following augmentation of dopaminergic neurotransmission due to inhibition of both dopamine-D3 and serotonin 5-HT2A receptors in the mPFC.
Similar content being viewed by others
INTRODUCTION
Schizophrenia is characterized by cognitive impairments as well as positive and negative symptoms (Freedman, 2003). Cognitive impairments are more pervasive than positive and negative symptoms, and fluctuate less over time, and they are strongly associated with poor psychosocial functioning (Spaulding et al, 1994). The impairments are thought to arise from dopaminergic and/or glutamatergic hypofunctioning that leads to hypostimulation of dopamine-D1 receptors and/or N-methyl-D-aspartate (NMDA) receptors, respectively, in the dorsolateral prefrontal cortex (dlPFC) of patients with schizophrenia (Javitt, 2007). The dlPFC in humans corresponds to the medial PFC (mPFC) of rodents (Brown and Bowman, 2002).
Blonanserin has a binding profile unique among the atypical antipsychotic drugs (APDs) in that it exhibits a higher affinity for dopamine-D2/3 receptors than for serotonin 5-HT2A receptors. In fact, the compound has been termed a dopamine–serotonin antagonist (DSA) (Tenjin et al, 2013). Clinically, blonanserin exhibits atypical APD properties, with efficacy against positive and negative symptoms and cognitive impairments (Tenjin et al, 2013). It is also generally well tolerated and appears to have an acceptable profile in terms of body-weight gain. Potential tolerability benefits of the drug in short-term trials with schizophrenia patients included fewer extrapyramidal symptoms (EPS) than with haloperidol (Garcia et al, 2009) and fewer reports of prolactin-level increases (hyperprolactinemia) than with risperidone (Takahashi et al, 2013). In animal studies, blonanserin has shown activity in reducing several psychobehavioral abnormalities in mice administered phencyclidine (PCP), a noncompetitive NMDA receptor antagonist that induces schizophrenia-like psychotomimetic states in humans and rodents (Castner et al, 2004; Javitt and Zukin, 1991; Morris et al, 2005; Noda et al, 2001; Noda et al, 1995). PCP-induced hyperlocomotion, which is considered a model of the positive symptoms of schizophrenia, was significantly antagonized by blonanserin. Potentiation of forced swimming-induced immobility by PCP, an animal model of schizophrenic negative symptoms, was also antagonized by blonanserin as well as by other atypical APDs (Nagai et al, 2003). Recently, blonanserin has shown to reverse the PCP-induced cognitive impairment in NORT due to indirect serotonin 5-HT1A receptor agonism in rats (Horiguchi and Meltzer, 2013).
The preclinical evidence suggests that dopamine-D3 receptors influence cognition by modulating the mPFC functions despite the relatively few dopamine-D3 receptors in this region (Nakajima et al, 2013). Unlike other atypical APDs, blonanserin has a high affinity for dopamine-D3 receptors (Tenjin et al, 2013) and enhances cognitive function in schizophrenia (Tenjin et al, 2012). However, the mechanism of the ameliorating effect of blonanserin on cognitive impairment remains unclear despite basic and clinic studies.
The aim of this study was to examine the effect of blonanserin on the impairment of visual-recognition memory induced in mice by repeated PCP administration and to further elucidate the involvement of dopamine-D3 and/or serotonin 5-HT2A receptors in this model. The study was also designed to analyze the postsynaptic mechanisms underlying the effect of blonanserin on impairment of visual-recognition memory. We found that blonanserin indirectly activates dopamine and NMDA receptors following augmentation of dopaminergic neurotransmission in the mPFC.
MATERIALS AND METHODS
Animals
Male mice of the ICR strain (Japan SLC, Shizuoka, Japan), aged 6 weeks at the beginning of the experiments, were used. The animals were housed in plastic cages kept in a regulated environment (22±2 °C, 55±10% humidity), with a 12:12 h light:dark cycle (lights on at 0800 hours). Food (CE2; CLEA Japan, Tokyo, Japan) and tap water were available ad libitum.
Behavioral experiments were performed in a sound-attenuated and air-regulated room, to which mice were habituated for at least 1 h. All experiments were conducted blind to treatment and in accordance with the Meijo University Faculty of Pharmacy Guidelines for Animal Experiments. The procedures involving animals and their care conformed to the international guidelines set out in the Principles of Laboratory Animal Care (National Institutes of Health publication no. 85–23, revised 1985).
Drugs
Blonanserin was supplied by Dainippon Sumitomo Pharma (Osaka, Japan). Olanzapine (Toronto Research Chemicals, Toronto, Canada), DOI (R(−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride; RBI-Funakoshi, Tokyo, Japan), 7-OH-DPAT ((±)-7-hydroxy N,N-di-n-propyl-2-aminotetralin hydrobromide; RBI-Funakoshi), SCH23390 (R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride; (RBI-Funakoshi), and H-89 (N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide; Sigma-Aldrich, St Louis, MO, USA) were used. PCP was synthesized by the authors according to the method described by Maddox et al (1965) and was checked for purity.
Blonanserin and olanzapine were initially dissolved in a minimum amount of 1.0 N HCl and diluted with saline. DOI, 7-OH-DPAT, SCH23390, H-89, and PCP were dissolved in saline.
Drug Treatments
The mice received saline or PCP (10 mg/kg/day, s.c.) once a day for 14 consecutive days (Noda et al, 1995). The novel-object recognition test (NORT) and microdialysis experiment were started 1 day and 3 days, respectively, after withdrawal of PCP administration. The saline- or PCP-administered mice were treated with blonanserin (1 or 3 mg/kg, p.o.) or olanzapine (1 or 3 mg/kg, p.o.) using a disposable feeding needle 30 min before the NORT training session or immediately after baseline collection in the microdialysis experiment. DOI (3 mg/kg, i.p.) was injected 25 min after treatment with blonanserin or olanzapine. 7-OH-DPAT (0.05 mg/kg, i.p.) and SCH23390 (0.02 mg/kg, s.c.) were injected 30 min before treatment with blonanserin or olanzapine. H-89 (0.03 or 0.1 nmol/μl) was bilaterally administered into the medial prefrontal cortex (mPFC: anteroposterior (AP): +1.7 mm, mediolateral (ML): ±0.5 mm from bregma, dorsoventral (DV): −2 mm from the skull) according to the atlas (Paxinos and Franklin, 1997), 30 min before the NORT training session. The doses of 7-OH-DPAT, used as a dopamine-D3 receptor agonist, were based on previous reports demonstrating activation of dopamine-D3 receptors at low doses and increased occupancy of dopamine-D2 receptors at higher doses (>0.3 mg/kg) (Ahlenius and Salmi, 1994; Daly and Waddington, 1993; Levant et al, 1996; Pritchard et al, 2003). The doses of blonanserin and olanzapine used in the present study were determined in previous experiments (Enomoto et al, 2005; Nagai et al, 2003). The doses of the other drugs were also based on previous publications (Mori et al, 1997; Nagai et al, 2003; Noda et al, 2010). All compounds except H-89 were systemically administered at a volume of 0.1 ml/10 g body weight. Control mice received the same volume of vehicle.
NORT
The task was carried out on days 1–5 after the final injection of PCP in accordance with the method described by Hida et al (2014), with a minor modification. The experimental apparatus consisted of a Plexiglas open-field box (30 cm × 30 cm × 35 cm), the floor of which was covered with paper bedding. The apparatus was placed in a sound-isolated room.
The NORT procedure consisted of three sessions: habituation, training, and retention. Each mouse was individually habituated to the box, with 10 min of exploration in the absence of objects on each of the 3 consecutive days (habituation sessions). During the training session on day 4, two objects (A and B) were symmetrically fixed to the floor of the box, 8 cm away from the sidewalls. The experimenter used a pair of stopwatches to record the time spent exploring each object. A mouse was then placed in the middle front of the box, and the total time spent in exploring the two objects was recorded for 10 min. Exploration of an object was defined as directing the nose to the object at a distance of <2 cm and/or touching it with the nose. After the training session, the mouse was immediately returned to its home cage. During the retention session on day 5, the mouse was returned to the same box 24 h after the training session, with one of the familiar objects (eg, A) used during the training session replaced by a novel object, C. The mouse was allowed to explore freely for 10 min, and the time spent exploring each object was recorded as before. Throughout the experiments, the objects were used in a counterbalanced manner in terms of their physical complexity and emotional neutrality. A preference index—the ratio of time spent exploring either of the two objects (training session) or the novel object (retention session) to the total amount of time spent exploring both objects—was used to assess cognitive function, eg, A or B/( B+A ) × 100 (%) in the training session, and B or C/( B+C ) × 100 in the retention session. To analyze locomotor activity, the floor of the open field was divided into 16 identical squares, and the number of times that a mouse crossed from one square to another over 10 min was recorded.
Microdialysis
On day 16 after the start of PCP administration (the second day after PCP withdrawal), the mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and fixed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). A guide cannula (AG-4; Eicom, Kyoto, Japan) was implanted in the mPFC (AP: +1.7 mm, ML: −0.3 mm from the bregma, DV: −1.5 mm from the skull) according to the atlas. On day 17 (24 h after implantation of the guide cannula and the third day after PCP withdrawal), a dialysis probe (A-I-4-02; membrane length 2 mm, Eicom) was implanted into the mPFC, and Ringer solution (147 mM NaCl, 4 mM KCl, and 2.3 mM CaCl2) was perfused at a flow rate of 1.0 μl/min. The dialysate was collected every 10 min, and the dopamine concentration was determined using an HPLC system (HTEC-500; Eicom) with electrochemical detection. Three samples were taken to establish baseline levels of extracellular dopamine. After treatment with blonanserin or olanzapine, dialysate was collected for a further 240 min, with Ringer perfusion as before.
Western Blotting Analysis
Western blotting was performed as previously described (Hida et al, 2014), with a minor modification. The mice were killed by decapitation after the NORT training session, and the brain immediately removed. The mPFC, hippocampus, and striatum samples were dissected out according to the atlas. Rabbit anti-phospho-PKA (Thr197) (1:1000; Cell Signaling), rabbit anti-PKA (1:1000; Cell Signaling), rabbit anti-phospho-NR1 (Ser897) (1:1000; Millipore), rabbit anti-phospho-NR1 (Ser896) (1:1000; Upstate Biotechnology), rabbit anti-NR1 (1:1000; Santa Cruz), and goat anti-β-actin (1:500, Santa Cruz Biotechnology) antibodies were used as primary antibodies. Horseradish peroxidase–conjugated anti-rabbit (1:2000; Kierkegaard & Perry Laboratories) and anti-goat IgG (1:1000; Kierkegaard & Perry Laboratories) were used as secondary antibodies. To evaluate PKA or NR1 activation, phosphorylated PKA or NR1 levels were normalized to total PKA or NR-1 levels on the same membranes, and then each phosphorylated/total level was normalized to the basal phosphorylated/total level of saline control mice.
Real-Time RT-PCR
Total RNA was isolated using a NucleoSpin RNA kit (Takara Bio, Otsu, Japan). Reverse transcription was performed with a PrimeScript RT reagent Kit (Perfect Real Time; Takara Bio) under the conditions recommended by the manufacturer. Real-time PCR analysis was undertaken using SYBR Premix Ex Taq (Takara Bio). Data were collected using a StepOnePlus System (Applied Biosystems, Foster City, CA, USA). The mouse NR1 primers used were as follows: 5′-ACTCCCAACGACCACTTCAC-3′ (forward) and 5′-GTAGACGCGCATCATCTCAA-3′ (reverse). Partial cDNA sequences of mouse NR1 have been deposited in the National Center for Biotechnology Information (NCBI) GenBank database (accession number: BC039157). All primers were purchased from Takara Bio. The real-time PCR conditions were as follows: amplification consisted of an initial step (95 °C for 30 s), 40 cycles of denaturation for 5 s at 95 °C, and annealing for 1 min at 60 °C. The expression levels of NR1 analyzed by real-time RT-PCR were quantified by comparison with a standard curve and normalized relative to the levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical Analysis
Statistical significance was determined using one-way analysis of variance (ANOVA) or two-way ANOVA with repeated measures, followed by Bonferroni’s test and Fisher’s PLSD test for multigroup comparisons. p-Values<0.05 were taken to indicate significance.
RESULTS
Effect of Blonanserin on the Impairment of Visual-Recognition Memory in Mice that Received Repeated PCP Administration, in Comparison with that of Olanzapine
We examined whether PCP-induced cognitive impairment was reversed by blonanserin as well as olanzapine, one of the atypical APDs. One day after PCP withdrawal, mice were subjected to the NORT. Blonanserin (1 and 3 mg/kg, p.o.) and olanzapine (1 and 3 mg/kg, p.o.) were treated acutely 30 min before the training session.
As shown in Figure 1a and c, repeated PCP administration significantly reduced the exploratory preference for a novel object in the retention session, compared with repeated saline administration. Treatment with blonanserin (3 mg/kg, p.o.) and olanzapine (3 mg/kg, p.o.) significantly ameliorated the cognitive impairment in PCP-administered mice without affecting exploratory preference in saline-administered mice (Figure 1a and c). In PCP-administered mice, neither blonanserin nor olanzapine affected exploratory preference between the objects in the training session. In addition, neither drug affected the total exploration time in either the training or the retention sessions (Figure 1b and d). There was no difference in the locomotor activity during the training session between PCP/vehicle- and PCP/blonanserin-administered mice or between saline/vehicle- and saline/blonanserin-administered mice (data not shown).
Effect of blonanserin on the impairment of visual-recognition memory in mice administered PCP repeatedly in comparison with that of olanzapine. Exploratory preference, (a and c); total exploration time, (b and d). PCP-administered mice were treated with blonanserin (a and b; 1 or 3 mg/kg, p.o.) or olanzapine (c and d; 1 or 3 mg/kg, p.o.) 30 min before the training session of the novel object recognition test (NORT). Exploratory preference was measured over a 10-min period. Values are mean±SEM (n=7–11). Two-way ANOVA: blonanserin: Sal: session: F1, 20=27.996, p<0.01; PCP: session: F1, 33=24.470, p<0.01, administration: F2, 33=10.414, p<0.01, (a); olanzapine: Sal: session: F1, 19=12.306, p<0.01; PCP: session: F1, 32=30.583, p<0.01, administration: F2, 32=8.335, p<0.01, session × administration: F2, 32=3.570, p<0.05, (c). Exploratory preference: one-way ANOVA: blonanserin: retention: F4, 35=5.851, p<0.01, (a); olanzapine: retention: F4, 33=4.364, p<0.01, (c). *p<0.05=significant difference compared with mice receiving saline, then vehicle (Sal/Veh). #p<0.05=significant difference compared with mice receiving PCP, then vehicle (PCP/Veh) (Bonferroni/Dunn test). BNS, blonanserin; OLZ, olanzapine; PCP, phencyclidine; Sal, saline; Veh, vehicle.
Involvement of Serotonin 5-HT2A and Dopamine-D3 Receptors in the Cognitive Amelioration Effect of Blonanserin in PCP-Administered Mice
Blonanserin has been demonstrated to have a high affinity not only for dopamine-D2 receptors but also for serotonin 5-HT2A and dopamine-D3 receptors (Tenjin et al, 2013). To determine whether the ameliorating effect of blonanserin on PCP-induced cognitive impairment involves serotonin 5-HT2A or dopamine-D3 receptors, we employed DOI, a serotonin 5-HT2A receptor agonist, and 7-OH-DPAT, a dopamine-D3 receptor agonist, by testing their effect on the cognitive amelioration induced by blonanserin in PCP-administered mice.
In the NORT training session, there was no difference in exploratory preference for the objects across all groups (Figure 2). DOI (3 mg/kg, i.p.) significantly and completely prevented the ameliorating effects of blonanserin and olanzapine on the impairment of visual-recognition memory in PCP-administered mice (Figure 2a and c). 7-OH-DPAT (0.05 mg/kg, i.p.) also significantly and completely prevented the ameliorating effect of blonanserin (Figure 2e), whereas it had no effect on that of olanzapine (Figure 2g).
Involvement of serotonin 5-HT2A and dopamine-D3 receptors in the mechanism by which blonanserin ameliorates cognitive impairment of PCP-administered mice. Exploratory preference, (a, c, e, and g); total exploration time, (b, d, f, and h). Mice were treated with DOI (3 mg/kg, i.p.) or 7-OH-DPAT (0.05 mg/kg, i.p.) 5 or 60 min before the NORT training session. One group received blonanserin (3 mg/kg, p.o.) and one group olanzapine (3 mg/kg, p.o.), both 30 min before the training session. Exploratory preference was measured over a 10-min period. Values indicate the mean±SEM (n=6–10). Two-way ANOVA: blonanserin/DOI: Sal: session: F1, 17=23.501, p<0.01; PCP: session: F1, 38=16.153, p<0.01, administration: F2, 38=7.222, p<0.01, (a); olanzapine/DOI: Sal: session: F1, 16=23.397, p<0.01; PCP: session: F1, 37=21.794, p<0.01, administration: F2, 37=10.620, p<0.01, session × administration: F2, 37=4.109, p<0.05, (c); blonanserin/7-OH-DPAT: Sal: session: F1, 17=16.890, p<0.01; PCP: session: F1, 28=16.363, p<0.01, administration: F2, 28=4.828, p<0.05, (e); olanzapine/7-OH-DPAT: Sal: session: F1, 13=33.049, p<0.01; PCP: session: F1, 25=33.067, p<0.01, administration: F2, 25=4.580, p<0.05, session × administration: F2, 25=5.049, p<0.05, (g). Exploratory preference: one-way ANOVA: blonanserin/DOI: retention: F4, 36=10.250, p<0.01, (a); olanzapine/DOI: retention: F4, 35=7.067, p<0.01, (c): blonanserin/7-OH-DPAT: retention: F4, 29=6.329, p<0.01, (e); olanzapine/7-OH-DPAT: retention: F4, 25=6.923, p<0.01, (g). *p<0.05 compared with mice receiving (Sal/Veh/Veh); #p<0.05 compared with mice receiving (PCP/Veh/Veh); $p<0.05 compared with mice receiving (PCP/BNS/Veh); †p<0.05 compared with mice receiving (PCP/OLZ/Veh) (Bonferroni/Dunn test). BNS, blonanserin; DPAT, 7-OH-DPAT; N.S., not significant; OLZ, olanzapine; PCP, phencyclidine; Sal, saline; Veh, vehicle.
In saline control mice, DOI and 7-OH-DPAT at the doses used had no effect on the novel-object recognition performances (Figure 2). The blocking effect of DOI and 7-OH-DPAT on blonanserin- and olanzapine-induced amelioration of exploratory preference reduction in PCP-administered mice was not associated with changes in the total exploration time (Figure 2b, d, f, and h).
Effect of Blonanserin on Extracellular Dopamine in the mPFC of PCP-Administered Mice: Involvement of Serotonin 5-HT2A and Dopamine-D3 Receptors
We have previously demonstrated that repeated PCP administration disables dopaminergic neurotransmission in the mPFC of mice, where such transmission is necessary for visual-recognition memory (Mouri et al, 2007; Nagai et al, 2009). We asked whether blonanserin and olanzapine, at a dose (3 mg/kg) that ameliorates cognitive impairment of PCP-administered mice, facilitate dopamine release in the mPFC of PCP-administered mice.
Our previous study demonstrated that the levels of extracellular dopamine decreased slightly in the mPFC of PCP-administered mice compared with saline-administered mice, and the change in the basal levels was mirrored in the dopaminergic response to potassium stimulation at a high concentration (Wang et al, 2007). There was no difference in the basal levels of extracellular dopamine in the mPFC of any PCP-administered groups in the present study (data not shown). As shown in Figure 3, blonanserin and olanzapine caused a marked increase in the levels of extracellular dopamine in the mPFC of PCP-administered mice (Figure 3). When DOI (3 mg/kg, i.p.) was administered 25 min after blonanserin or olanzapine treatment, increases in the levels of extracellular dopamine induced by either drug were significantly diminished (Figure 3a and b). 7-OH-DPAT (0.05 mg/kg, i.p.) also significantly inhibited blonanserin-induced increases in the levels of extracellular dopamine (Figure 3c), but it did not inhibit the dopamine-increasing effect of olanzapine (Figure 3d). 7-OH-DPAT or DOI in the absence of any APD did not affect the levels of extracellular dopamine in the mPFC (Figure 3a–d).
Effect of blonanserin on the level of extracellular dopamine in the medial prefrontal cortex (mPFC) of PCP-administered mice. PCP-administered mice were treated with DOI (3 mg/kg, i.p.) (a and b) or 7-OH-DPAT (0.05 mg/kg, i.p.) (c and d) 25 min after (DOI) or 30 min before (7-OH-DPAT), blonanserin (3 mg/kg, p.o.), or olanzapine (3 mg/kg, p.o.) treatments. Values indicate the mean±SEM (n=4–5). Two-way ANOVA: blonanserin/DOI: administration: F2, 10=48.389, p<0.01, administration × time: F48, 240=1.914, p<0.01, (a); olanzapine/DOI: administration: F2, 9=5.554, p<0.05, administration × time: F48, 216=2.526, p<0.01, (b); blonanserin/7-OH-DPAT: administration: F2, 10=5.217, p<0.05, administration × time: F48, 240=2.034, p<0.01, (c); olanzapine/7-OH-DPAT: administration × time: F48, 216=2.311, p<0.01, (d). **p<0.01 compared with mice receiving (PCP/BNS/Veh); ##p<0.01 compared with mice receiving (PCP/OLZ/Veh); $$p<0.01 compared with mice receiving (PCP/Veh/DPAT) (Bonferroni/Dunn test). BNS, blonanserin; DPAT, 7-OH-DPAT; OLZ, olanzapine; PCP, phencyclidine; Veh, vehicle.
Involvement of Intracellular Signaling through Dopamine-D1 Receptors in Cognitive Amelioration by Blonanserin in PCP-Administered Mice
Previous studies have shown that when repeated PCP administration induces cognitive impairment in mice, the impairment is accompanied by dysfunction of the dopamine-D1 receptors in the mPFC (Mouri et al, 2007). We asked whether the ameliorating effect of blonanserin follows from the increased levels of extracellular dopamine the APD induces in the mPFC, acting through activation of dopamine-D1 receptor to PKA signaling.
SCH23390 (0.02 mg/kg, s.c.), a dopamine-D1 receptor antagonist, and H-89 (0.1 nmol/μl, bilaterally), a PKA inhibitor, significantly and completely prevented the ameliorating effect of blonanserin on PCP-induced cognitive impairment (Figure 4a and e) but not H-89 (0.03 nmol/μl) (Figure 4c). Exploratory preference across objects during the training session was unaffected. In saline control mice, SCH23390 and H-89 had no effect on novel-object recognition performance (Figure 4a, c and e). In PCP-administered mice, SCH23390 and H-89 also had no effect on the total exploration time in either the training or the retention sessions (Figure 4b, d and f).
Involvement of intracellular signaling through dopamine-D1 receptors in the mechanism by which blonanserin ameliorates the cognitive impairment of PCP-administered mice. Exploratory preference, (a, c, and e); total exploration time, (b, d, and f). PCP-administered mice were treated with SCH23390 (0.02 mg/kg, s.c.), H-89 (0.03 or 0.1 nmol/μl), and blonanserin (3 mg/kg, p.o.) 60, 30, and 30 min, respectively, before the NORT training session. Exploratory preference was measured over a 10-min period. Values indicate the mean±SEM (n=5–8). Two-way ANOVA: blonanserin/SCH23390: Sal: session: F1, 11=9.068, p<0.05; PCP: administration: F2, 21=9.451, p<0.01, session × administration: F2, 21=4.959, p<0.05; (a); blonanserin/H-89 (0.03): Sal: session: F1, 21=22.757, p<0.01; PCP: session: F1, 59=22.865, p<0.01, administration: F2, 59=7.086, p<0.01; (c); blonanserin/H-89 (0.1): Sal: session: F1, 15=8.126, p<0.05; PCP: administration: F2, 28=4.576, p<0.05, session × administration: F2, 28=5.614, p<0.01; (e). One-way ANOVA: exploratory preference: blonanserin/SCH23390: retention: F4, 21=9.032, p<0.01, (a): blonanserin/H-89 (0.03): retention: F4, 51=8.728, p<0.01, (c): blonanserin/H-89 (0.1): retention: F4, 27=9.483, p<0.01, (e). *p<0.05 compared with mice receiving (Sal/Veh/Veh); #p<0.05 compared with mice receiving (PCP/Veh/Veh); $p<0.05 compared with mice receiving (PCP/BNS/Veh) (Bonferroni/Dunn test). BNS, blonanserin; N.S., not significant; PCP, phencyclidine; Sal, saline; SCH, SCH23390; Veh, vehicle.
The phospholyrated levels of PKA at Thr197 in the PCP-administered mice were significantly decreased in the mPFC compared with those in the saline control mice (Figure 5a) but not in the hippocampus or striatum (Supplementary Figure S1a and c). Blonanserin (3 mg/kg) significantly remediated the decrease in phosphorylation levels, and this effect was significantly blocked by SCH23390 in the mPFC (Figure 5a). There was no significant groupwise variation in the levels of total PKA (Figure 5b, Supplementary Figure S1b and d).
Effect of blonanserin on Thr197-phosphorylated PKA and Ser897- or Ser896-phosphorylated NR1 in the medial prefrontal cortex (mPFC) of PCP-administered mice. PCP-administered mice were treated with SCH23390 (0.02 mg/kg, s.c.) and blonanserin (3 mg/kg, p.o.) for 60 and 30 min, respectively, before the NORT training session. Immediately after the training trial, mice were decapitated, and then Thr197-phosphorylated PKA (a) or NR1 (c, Ser897; d, Ser896), and total PKA (b) or NR1 (e) expression in the mPFC were determined by western blotting analysis. Representative western blot and phosphorylated PKA/total PKA or phosphorylated NR1/total NR1 immunoreactivity in the mPFC. Values indicate the mean±SEM (n=5–9). One-way ANOVA: PKA: F3, 32=4.046, p<0.01, (a); NR1 Ser897: F3, 19=4.046, p<0.05, (c). *p<0.05 compared with mice receiving (Sal/Veh/Veh); #p<0.05, ##p<0.01 compared with mice receiving (PCP/Veh/Veh); $$p<0.01 compared with mice receiving (PCP/BNS/Veh) (Fisher's PLSD test). BNS, blonanserin; PCP, phencyclidine; Sal, saline; SCH, SCH23390; Veh, vehicle.
It is well known that PKA phosphorylates NR1, an essential subunit of NMDA receptors, at Ser897 and regulates its functions (Mouri et al, 2007). To examine further the mechanism by which blonanserin ameliorates the impairment of visual-recognition memory in PCP-administered mice, we examined the effect of blonanserin on the Ser897-phosphorylation level of NR1 in the mPFC, hippocampus, and striatum of PCP-administered mice immediately after the training session. Without blonanserin, the Ser897-phosphorylation levels at this point were significantly decreased compared with the saline control mice in the mPFC (Figure 5c) but not the hippocampus or striatum (Supplementary Figure S2a and c). Blonanserin (3 mg/kg) significantly remediated the decrease in phosphorylation levels, and this effect was significantly blocked by SCH23390 in the mPFC (Figure 5c). However, there was no significant difference in the levels of NR1 phosphorylated at Ser896 (as opposed to Ser897), which is phosphorylated by PKC (as opposed to PKA), in the mPFC (Figure 5d), hippocampus, or striatum (data not shown) across all groups. There was no significant groupwise variation in the levels of total NR1 (Figure 5e, Supplementary Figure S2b and d). The mRNA levels of NR1 were also not significantly diferrent in the mPFC, hippocampus, or striatum (Supplementary Figure S3).
DISCUSSION
We have reconfirmed that PCP-administered mice show impaired novelty discrimination in the NORT that is consistent with previous reports (Nagai et al, 2009). It is unlikely that the impairment these mice exhibit in learning and memory tasks is due to changes in motivation relative to the controls, although various motivational processes are involved in the behavioral task. The fact that PCP reduced the exploratory preference for novel objects in the retention session could be interpreted as neophobia. However, involvement of motivation and/or neophobia can be excluded, because PCP administration had no effect on total time devoted to exploration of novel objects during the training session. Therefore it is likely that the performance impairment seen in PCP-administered mice is due to learning and/or memory deficits.
Blonanserin inhibits serotonin 5-HT2A and dopamine-D2/3 receptors (Tenjin et al, 2013). Blonanserin significantly ameliorated the cognitive impairment induced by PCP as seen in the NORT, but at a dose of 3 mg/kg it had no effect on total exploration time recorded during the training session. It is unlikely that the observed amelioration of task-performance impairment was due to changes in motivation in PCP-administered mice. Blonanserin also had no effect on performance, such as augmentation of visual-recognition memory by itself in saline-administered mice. Similar phenomena were observed in olanzapine-treated, saline-, and PCP-administered mice. Therefore, it appears that blonanserin and olanzapine ameliorate the impairment of visual-recognition memory caused by repeated PCP administration in mice. In addition, the effect of blonanserin on impairment of visual-recognition memory may be due to an effect in acquisition (‘learning’), not consolidation or retention (‘memory’), because the PCP-administered mice were treated with blonanserin before the training, not after the training or before the test sessions.
The ameliorating effect of blonanserin on impairment of visual-recognition memory was prevented by treatment with DOI, a serotonin 5-HT2A receptor agonist, and 7-OH-DPAT, a dopamine-D3 receptor agonist, at doses that did not significantly affect the performance of the saline control mice. Our findings with 7-OH-DPAT should be interpreted in the context of the known selectivity of 7-OH-DPAT at the dopamine-D3 vs -D2 receptors. We had already found that haloperidol, a dopamine-D2 receptor antagonist, failed to reverse impairment of visual-recognition memory in PCP-administered mice (Nagai et al, 2009), indicating no association of dopamine-D2 receptor antagonism with amelioration of the PCP-induced impairment. We demonstrated that the results with 7-OH-DPAT prevented the ameliorating effect of blonanserin through the dopamine-D3 receptors. Olanzapine also ameliorated PCP-induced impairment of visual-recognition memory; however, the effect was canceled only by DOI, not by 7-OH-DPAT. From the present results, and reports that blonanserin has a higher affinity for dopamine-D3 receptors than olanzapine (Ki=0.49 nM vs 49 nM) (DeLeon et al, 2004; Tenjin et al, 2013), our conclusion is that blonanserin ameliorates PCP-induced cognitive impairment by antagonizing both serotonin 5-HT2A and dopamine-D3 receptors and shows a pharmacological efficacy different from that of olanzapine.
On the other hand, subeffective dose of tandospirone potentiated the ability of blonanserin to counteract the PCP-induced cognitive impairment (Horiguchi and Meltzer, 2013), although blonanserin itself has low affinity for serotonin 5-HT1A receptors (Ki=804 nM; Tenjin et al, 2013). It cannot be ruled out that, despite its markedly lower affinity for serotonin 5-HT1A receptors than for serotonin 5-HT2A/dopamine-D3 receptors in binding studies, blonanserin may stimulate serotonin 5-HT1A receptors via an allosteric or other mechanism (Horiguchi and Meltzer, 2013). Further studies will be needed to clarify the functional relationship between serotonin 5-HT2A/dopamine-D3 and 5-HT1A receptors in the ameliorating effect of blonanserin on cognitive impairment.
Accumulating evidence suggests that the dopaminergic system in the mPFC is involved in cognitive function. For instance, disruption of dopaminergic neurotransmission in the mPFC of nonhuman primates by infusion of dopamine-D1 receptor antagonists or by excitotoxic lesion impairs the performance of object retrieval-detour and delayed-response tasks (Dias et al, 1996a, 1996b; Sawaguchi and Goldman-Rakic, 1991). Accordingly, cognitive impairments in schizophrenia may be associated with deficits in dopaminergic neurotransmission in the mPFC. Serotonin 5-HT2A receptors are widely distributed in the brain, with their highest concentrations in the cortex (Hoyer et al, 1986; Meltzer et al, 2003). The atypical APDs, represented by olanzapine and risperidone, facilitate dopamine release in the mPFC, striatum, and hypophysis through antagonism of serotonin 5-HT2A receptors. The facilitating effects of atypical APDs on dopamine release in these regions are critical for the amelioration of cognitive impairments, negative symptoms, and side effects of APDs, such as EPS and hyperprolactinemia (Meltzer and McGurk, 1999). The previous and present in vivo microdialysis experiments showed that the basal extracellular dopamine levels in the mPFC of PCP-administered mice were slightly decreased (Wang et al, 2007) and that both blonanserin and olanzapine significantly increased dopamine release in the mPFC of PCP-administered mice at the same dose that ameliorated cognitive impairment. These dopamine-release effects of blonanserin and olanzapine were antagonized by DOI. The findings suggest that the antagonism of serotonin 5-HT2A receptors by blonanserin and olanzapine is at least partly responsible, via facilitation of dopamine release in the mPFC, for the amelioration of PCP-induced cognitive impairment.
On the other hand, dopamine-D3 receptors have a dual role as autoreceptors and postsynaptic receptors at dopaminergic synapses (Diaz et al, 1995; Diaz et al, 2000; Levesque et al, 1992). Pharmacological studies suggest that dopamine-D3 receptors acting as autoreceptors negatively modulate dopamine release (Gross and Drescher, 2012). Ohno et al (2010) found that AD-6048, a primary metabolite of blonanserin, shows a higher affinity for dopamine-D3 receptors than for dopamine-D2 receptors, which at least partly explains the atypical nature of blonanserin and its low EPS liability. In fact, selective dopamine-D3 receptor antagonists are known to attenuate APD-induced EPS, and APDs that activate mesolimbic dopaminergic systems show reduced EPS liability (Gyertyan and Saghy, 2007; Gyertyan et al, 2008; Millan et al, 1997; Perrault et al, 1997). Antagonism of dopamine-D3, but not D2, receptors can also enhance cortical cognitive function by facilitating the release and synthesis of dopamine by the mesocortical dopaminergic system (Gobert et al, 1995; Gross and Drescher, 2012; Nakajima et al, 2013; Watson et al 2012a, 2012b). We found that the disinhibiting effect of blonanserin, but not of olanzapine, on dopamine release was antagonized by 7-OH-DPAT at the same dose that antagonized the ameliorating effect of blonanserin on cognitive impairment. These results strongly suggest that blonanserin ameliorates PCP-induced impairment of visual-recognition memory by antagonizing dopamine-D3 receptors, in addition to serotonin 5-HT2A receptors, thereby stimulating the release of dopamine in the mPFC.
Previous studies have shown that the cognitive impairments in PCP-administered mice are accompanied by dysfunction of the dopamine-D1 (but not dopamine-D2) and/or NMDA receptors in the mPFC (Abekawa et al, 2006). Coimmunoprecipitation studies using homogenates from the mPFC as well as the hippocampus have demonstrated that dopamine-D1 receptors are close enough to the NR1 subunit to affect its activity (Kruse et al, 2009). In vitro physiological studies using pyramidal cells from the mPFC of rats have demonstrated that NMDA receptor functions are facilitated by a dopamine-D1 receptor agonist, SKF38393, and the facilitation is dependent on PKA and intracellular calcium (Tseng and O'Donnell, 2004). In the present study, the ameliorating effect of blonanserin on PCP-induced cognitive impairment was completely blocked by pretreatment with a dopamine-D1 receptor antagonist, SCH23390, or a PKA inhibitor, H-89, at doses not affecting the performance of saline control mice. It is unlikely that the relatively high affinity of SCH23390 for serotonin 5-HT2A receptors has a role in its antagonism, because (+) SCH23390 at the doses used in the present study fail to affect the in vivo binding of [3H]spiperone in the rat prefrontal cortex (Bischoff et al, 1986). That is, SCH23390 selectively antagonizes dopamine-D1 receptors, enabling it to act independently of serotonin 5-HT2A receptors. Blonanserin significantly remediated the decrease in the levels of Thr197-phosphorylated PKA, and this effect was significantly blocked by SCH23390 in the mPFC of PCP-administered mice. Accordingly, these results suggest that the ameliorating effect of blonanserin on PCP-induced cognitive impairment is related to the activation of dopamine-D1 receptor-PKA signaling in the mPFC. In the future, however, more direct investigation to detect PKA enzyme activity is needed to determine the relationship between the phosphorylated form and enzyme activity of PKA. The abnormally decreased levels of Ser897-phosphorylated NR1, which is phosphorylated by PKA, in the mPFC of the PCP-administered mice were significantly increased by blonanserin, the effect being blocked by SCH23390. There was no significant difference in the levels of Ser896-phosphorylated NR1, which is phosphorylated by PKC rather than PKA, in the mPFC in any group. The detected effects were not observed in the hippocampus or striatum. Further experiments are needed to clarify the brain region–specific effect of blonanserin, because it might be caused by the difference in neuronal projections or receptor expressions and/or sensitivity to effects of blonanserin in each brain region (Gurevich and Joyce, 1999; Marek, 2007). Our findings suggest that dopamine-D1 receptor-PKA signaling is required for the ameliorating effect of blonanserin on cognitive impairment. In addition, activation of NMDA receptors in the mPFC through dopamine-D1 receptor-PKA, but not PKC, signaling is critical for visual-recognition memory in PCP-administered mice.
In conclusion, the ameliorating effect of blonanserin on PCP-induced cognitive impairment is associated with indirect activation of NMDA receptors due to Ser897-phosphorylation of the NR1 subunit, a step linked to dopamine-D1 receptor-PKA signaling following facilitation of dopamine release in vivo. The latter is due to a dual antagonism of serotonin 5-HT2A and dopamine-D3 receptors by blonanserin. These findings also provide in vivo evidence that blonanserin augments dopaminergic neurotransmission in the mPFC through antagonizing of dopamine-D3 receptors.
FUNDING AND DISCLOSURE
This study was supported by the ‘Academic Frontier’ Project for Private Universities (2007–2011) and Grants-in-Aid for Scientific Research C (24590219 and 26460240) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan; Research on Risk of Chemical Substances (2008–2010), Health and Labor Science Research Grants supported by the Ministry of Health, Labor and Welfare (MHLW); a Meijo University Research Institute grant; a Smoking Research Foundation Grant for Biomedical Research (SRF); the Joint Research Project under the Japan–Korea basic scientific cooperation program (2010–2012) from the Japan Society for the Promotion of Science (JSPS); the Adaptable and Seamless Technology Transfer Program Through Target-driven R&D (AS251Z02986P, AS251Z03018Q), Japan Science and Technology Agency; a Grant-in-Aid for the Japan Society for the Promotion of Science (JSPS) Fellows; and a Grant-in-Aid for Scientific Research on Innovative Areas, ‘Glial Assembly: A New Regulatory Machinery of Brain Function And Disorders’, and a Grant-in-Aid for ‘Integrated Research on Neuropsychiatric Disorders’ carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Dr Y Noda has received research support and speakers' honoraria from Dainippon Sumitomo. Dr N Ozaki has received research support or speakers’ honoraria from, or has served as a consultant to, Abbvie, Asahi Kasei Pharma, Astellas, Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Meiji Seika Pharma, Mochida, MSD, Novartis Pharma, Ono, Otsuka, Pfizer, Shionogi, Takeda, Tanabe Mitsubishi, Sanofi, and Yoshitomi. Dr K Iwamoto has received speakers’ honoraria from Dainippon Sumitomo, Eisai, Eli Lilly, GlaxoSmithKline, Meiji Seika Pharma, Mochida, Otsuka, and Tanabe Mitsubishi. The other authors declare no conflict of interest.
Accession codes
References
Abekawa T, Ito K, Koyama T (2006). Role of the simultaneous enhancement of NMDA and dopamine D1 receptor-mediated neurotransmission in the effects of clozapine on phencyclidine-induced acute increases in glutamate levels in the rat medial prefrontal cortex. Naunyn Schmiedebergs Arch Pharmacol 374: 177–193.
Ahlenius S, Salmi P (1994). Behavioral and biochemical effects of the dopamine D3 receptor-selective ligand, 7-OH-DPAT, in the normal and the reserpine-treated rat. Eur J Pharmacol 260: 177–181.
Bischoff S, Heinrich M, Sonntag JM, Krauss J (1986). The D1 dopamine receptor antagonist SCH 23390 also interacts potently with brain serotonin (5-HT2) receptors. Eur J Pharmacol 129: 367–370.
Brown VJ, Bowman EM (2002). Rodent models of prefrontal cortical function. Trends Neurosci 25: 340–343.
Castner SA, Goldman-Rakic PS, Williams GV (2004). Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology (Berl) 174: 111–125.
Daly SA, Waddington JL (1993). Behavioural effects of the putative D3 dopamine receptor agonist 7-OH-DPAT in relation to other ‘D2-like’ agonists. Neuropharmacology 32: 509–510.
DeLeon A, Patel NC, Crismon ML (2004). Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther 26: 649–666.
Dias R, Robbins TW, Roberts AC (1996a). Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380: 69–72.
Dias R, Robbins TW, Roberts AC (1996b). Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110: 872–886.
Diaz J, Levesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC et al (1995). Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 65: 731–745.
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC et al (2000). Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20: 8677–8684.
Enomoto T, Noda Y, Mouri A, Shin EJ, Wang D, Murai R et al (2005). Long-lasting impairment of associative learning is correlated with a dysfunction of N-methyl-D-aspartate-extracellular signaling-regulated kinase signaling in mice after withdrawal from repeated administration of phencyclidine. Mol Pharmacol 68: 1765–1774.
Freedman R (2003). Schizophrenia. N Engl J Med 349: 1738–1749.
Garcia E, Robert M, Peris F, Nakamura H, Sato N, Terazawa Y (2009). The efficacy and safety of blonanserin compared with haloperidol in acute-phase schizophrenia: a randomized, double-blind, placebo-controlled, multicentre study. CNS Drugs 23: 615–625.
Gobert A, Rivet JM, Audinot V, Cistarelli L, Spedding M, Vian J et al (1995). Functional correlates of dopamine D3 receptor activation in the rat in vivo and their modulation by the selective antagonist, (+)-S14297: II. Both D2 and ‘silent’ D3 autoreceptors control synthesis and release in mesolimbic, mesocortical and nigrostriatal pathways. J Pharmacol Exp Ther 275: 899–913.
Gross G, Drescher K (2012). The role of dopamine D3 receptors in antipsychotic activity and cognitive functions. Handb Exp Pharmacol 213: 167–210.
Gurevich EV, Joyce JN (1999). Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20: 60–80.
Gyertyan I, Saghy K (2007). The selective dopamine D3 receptor antagonists, SB277011-A and S33084 block haloperidol-induced catalepsy in rats. Eur J Pharmacol 572: 171–174.
Gyertyan I, Saghy K, Laszy J, Elekes O, Kedves R, Gemesi LI et al (2008). Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: II. behavioural characterisation of RG-15. Naunyn Schmiedebergs Arch Pharmacol 378: 529–539.
Hida H, Mouri A, Ando Y, Mori K, Mamiya T, Iwamoto K et al (2014). Combination of neonatal PolyI:C and adolescent phencyclidine treatments is required to induce behavioral abnormalities with overexpression of GLAST in adult mice. Behav Brain Res 258: 34–42.
Horiguchi M, Meltzer HY (2013). Blonanserin reverses the phencyclidine (PCP)-induced impairment in novel object recognition (NOR) in rats: role of indirect 5-HT1A partial agonism. Behav Brain Res 247: 158–164.
Hoyer D, Pazos A, Probst A, Palacios JM (1986). Serotonin receptors in the human brain. II. Characterization and autoradiographic localization of 5-HT1C and 5-HT2 recognition sites. Brain Res 376: 97–107.
Javitt DC (2007). Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 78: 69–108.
Javitt DC, Zukin SR (1991). Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301–1308.
Kruse MS, Premont J, Krebs MO, Jay TM (2009). Interaction of dopamine D1 with NMDA NR1 receptors in rat prefrontal cortex. Eur Neuropsychopharmacol 19: 296–304.
Levant B, Bancroft GN, Selkirk CM (1996). In vivo occupancy of D2 dopamine receptors by 7-OH-DPAT. Synapse 24: 60–64.
Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E et al (1992). Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA 89: 8155–8159.
Maddox VH, Godefroi EF, Parcell RF (1965). The synthesis of phencyclidine and other 1-arylcyclohexylamines. J Med Chem 8: 230–235.
Marek GJ (2007). Serotonin and dopamine interactions in rodents and primates: implications for psychosis and antipsychotic drug development. Int Rev Neurobiol 78: 165–192.
Meltzer HY, Li Z, Kaneda Y, Ichikawa J (2003). Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 27: 1159–1172.
Meltzer HY, McGurk SR (1999). The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25: 233–255.
Millan MJ, Gressier H, Brocco M (1997). The dopamine D3 receptor antagonist, (+)-S14297, blocks the cataleptic properties of haloperidol in rats. Eur J Pharmacol 321: R7–R9.
Mori T, Murase K, Tanaka J, Ichimaru Y (1997). Biphasic effects of D3-receptor agonists, 7-OH-DPAT and PD128907, on the D1-receptor agonist-induced hyperactivity in mice. Jpn J Pharmacol 73: 251–254.
Morris BJ, Cochran SM, Pratt JA (2005). PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol 5: 101–106.
Mouri A, Noda Y, Noda A, Nakamura T, Tokura T, Yura Y et al (2007). Involvement of a dysfunctional dopamine-D1/N-methyl-d-aspartate-NR1 and Ca2+/calmodulin-dependent protein kinase II pathway in the impairment of latent learning in a model of schizophrenia induced by phencyclidine. Mol Pharmacol 71: 1598–1609.
Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H et al (2009). Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology (Berl) 202: 315–328.
Nagai T, Noda Y, Une T, Furukawa K, Furukawa H, Kan QM et al (2003). Effect of AD-5423 on animal models of schizophrenia: phencyclidine-induced behavioral changes in mice. Neuroreport 14: 269–272.
Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foll B et al (2013). The potential role of dopamine D3 receptor neurotransmission in cognition. Eur Neuropsychopharmacol 23: 799–813.
Noda A, Noda Y, Kamei H, Ichihara K, Mamiya T, Nagai T et al (2001). Phencyclidine impairs latent learning in mice: interaction between glutamatergic systems and sigma1 receptors. Neuropsychopharmacology 24: 451–460.
Noda Y, Mouri A, Ando Y, Waki Y, Yamada SN, Yoshimi A et al (2010). Galantamine ameliorates the impairment of recognition memory in mice repeatedly treated with methamphetamine: involvement of allosteric potentiation of nicotinic acetylcholine receptors and dopaminergic-ERK1/2 systems. Int J Neuropsychopharmacol 13: 1343–1354.
Noda Y, Yamada K, Furukawa H, Nabeshima T (1995). Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol 116: 2531–2537.
Ohno Y, Okano M, Imaki J, Tatara A, Okumura T, Shimizu S (2010). Atypical antipsychotic properties of blonanserin, a novel dopamine D2 and 5-HT2A antagonist. Pharmacol Biochem Behav 96: 175–180.
Paxinos G, Franklin K (1997) The Mouse Brain in Stereotaxic Coordinates. Academic Press: New York, NY, USA, 360 pp.
Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B (1997). Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther 280: 73–82.
Pritchard LM, Logue AD, Hayes S, Welge JA, Xu M, Zhang J et al (2003). 7-OH-DPAT and PD128907 selectively activate the D3 dopamine receptor in a novel environment. Neuropsychopharmacology 28: 100–107.
Sawaguchi T, Goldman-Rakic PS (1991). D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251: 947–950.
Spaulding WD, Sullivan M, Weiler M, Reed D, Richardson C, Storzbach D (1994). Changing cognitive functioning in rehabilitation of schizophrenia. Acta Psychiatr Scand Suppl 384: 116–124.
Takahashi S, Suzuki M, Uchiyama M (2013). One-year follow-up study of psychotic patients treated with blonanserin: a case series. Asia Pac Psychiatry 5: 164–167.
Tenjin T, Miyamoto S, Miyake N, Ogino S, Kitajima R, Ojima K et al (2012). Effect of blonanserin on cognitive function in antipsychotic-naive first-episode schizophrenia. Hum Psychopharmacol 27: 90–100.
Tenjin T, Miyamoto S, Ninomiya Y, Kitajima R, Ogino S, Miyake N et al (2013). Profile of blonanserin for the treatment of schizophrenia. Neuropsychiatr Dis Treat 9: 587–594.
Tseng KY, O'Donnell P (2004). Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci 24: 5131–5139.
Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, Nabeshima T (2007). Synergistic effect of combined treatment with risperidone and galantamine on phencyclidine-induced impairment of latent visuospatial learning and memory: Role of nAChR activation-dependent increase of dopamine D1 receptor-mediated neurotransmission. Neuropharmacology 53: 379–389.
Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC (2012a). Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology 37: 770–786.
Watson DJ, Marsden CA, Millan MJ, Fone KC (2012b). Blockade of dopamine D3 but not D2 receptors reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int J Neuropsychopharmacol 15: 471–484.
Acknowledgements
The authors thank Mayu Ukai, Yurie Ogino, Fumiya Yamamoto, and Yuri Sakakibara of the Division of Clinical Sciences and Neuropsychopharmacology, Meijo University, Faculty of Pharmacy, for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Hida, H., Mouri, A., Mori, K. et al. Blonanserin Ameliorates Phencyclidine-Induced Visual-Recognition Memory Deficits: the Complex Mechanism of Blonanserin Action Involving D3-5-HT2A and D1-NMDA Receptors in the mPFC. Neuropsychopharmacol 40, 601–613 (2015). https://doi.org/10.1038/npp.2014.207
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.207
This article is cited by
-
HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury
Journal of Neuroinflammation (2021)
-
The epistatic interaction between the dopamine D3 receptor and dysbindin-1 modulates higher-order cognitive functions in mice and humans
Molecular Psychiatry (2021)
-
25C-NBF, a new psychoactive substance, has addictive and neurotoxic potential in rodents
Archives of Toxicology (2020)
-
Protein Kinase Cδ Gene Depletion Protects Against Methamphetamine-Induced Impairments in Recognition Memory and ERK1/2 Signaling via Upregulation of Glutathione Peroxidase-1 Gene
Molecular Neurobiology (2017)
-
Adolescent stress leads to glutamatergic disturbance through dopaminergic abnormalities in the prefrontal cortex of genetically vulnerable mice
Psychopharmacology (2017)