Abstract
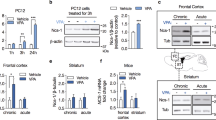
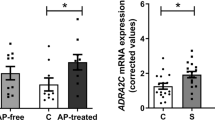
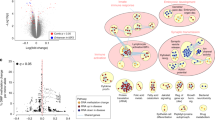
Histone deacetylases (HDACs) compact chromatin structure and repress gene transcription. In schizophrenia, clinical studies demonstrate that HDAC inhibitors are efficacious when given in combination with atypical antipsychotics. However, the molecular mechanism that integrates a better response to antipsychotics with changes in chromatin structure remains unknown. Here we found that chronic atypical antipsychotics downregulated the transcription of metabotropic glutamate 2 receptor (mGlu2, also known as Grm2), an effect that was associated with decreased histone acetylation at its promoter in mouse and human frontal cortex. This epigenetic change occurred in concert with a serotonin 5-HT2A receptor–dependent upregulation and increased binding of HDAC2 to the mGlu2 promoter. Virally mediated overexpression of HDAC2 in frontal cortex decreased mGlu2 transcription and its electrophysiological properties, thereby increasing psychosis-like behavior. Conversely, HDAC inhibitors prevented the repressive histone modifications induced at the mGlu2 promoter by atypical antipsychotics, and augmented their therapeutic-like effects. These observations support the view of HDAC2 as a promising new target for schizophrenia treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sawa, A. & Snyder, S.H. Schizophrenia: diverse approaches to a complex disease. Science 296, 692–695 (2002).
Dobbs, D. Schizophrenia: the making of a troubled mind. Nature 468, 154–156 (2010).
Lieberman, J.A. et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 353, 1209–1223 (2005).
Lieberman, J.A. et al. Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol. Rev. 60, 358–403 (2008).
Van Sant, S.P. & Buckley, P.F. Pharmacotherapy for treatment-refractory schizophrenia. Expert Opin. Pharmacother. 12, 411–434 (2011).
Dong, E., Guidotti, A., Grayson, D.R. & Costa, E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc. Natl. Acad. Sci. USA 104, 4676–4681 (2007).
Dong, E., Nelson, M., Grayson, D.R., Costa, E. & Guidotti, A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc. Natl. Acad. Sci. USA 105, 13614–13619 (2008).
Abel, T. & Zukin, R.S. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr. Opin. Pharmacol. 8, 57–64 (2008).
Citrome, L. et al. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatr. Serv. 55, 290–294 (2004).
Kelly, D.L. et al. Adjunct divalproex or lithium to clozapine in treatment-resistant schizophrenia. Psychiatr. Q. 77, 81–95 (2006).
Suzuki, T. et al. Augmentation of atypical antipsychotics with valproic acid. An open-label study for most difficult patients with schizophrenia. Hum. Psychopharmacol. 24, 628–638 (2009).
Löscher, W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog. Neurobiol. 58, 31–59 (1999).
Gurvich, N., Tsygankova, O.M., Meinkoth, J.L. & Klein, P.S. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 64, 1079–1086 (2004).
Borrelli, E., Nestler, E.J., Allis, C.D. & Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 60, 961–974 (2008).
Arrowsmith, C.H., Bountra, C., Fish, P.V., Lee, K. & Schapira, M. Epigenetic protein families: a new frontier for drug discovery. Nat. Rev. Drug Discov. 11, 384–400 (2012).
Miyamoto, S., Duncan, G.E., Marx, C.E. & Lieberman, J.A. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 10, 79–104 (2005).
González-Maeso, J. & Sealfon, S.C. Psychedelics and schizophrenia. Trends Neurosci. 32, 225–232 (2009).
Vollenweider, F.X., Vollenweider-Scherpenhuyzen, M.F., Babler, A., Vogel, H. & Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9, 3897–3902 (1998).
González-Maeso, J. et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53, 439–452 (2007).
Moreno, J.L., Sealfon, S.C. & González-Maeso, J. Group II metabotropic glutamate receptors and schizophrenia. Cell. Mol. Life Sci. 66, 3777–3785 (2009).
Fell, M.J., Svensson, K.A., Johnson, B.G. & Schoepp, D.D. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039). J. Pharmacol. Exp. Ther. 326, 209–217 (2008).
Woolley, M.L., Pemberton, D.J., Bate, S., Corti, C. & Jones, D.N. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl.) 196, 431–440 (2008).
Fribourg, M. et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147, 1011–1023 (2011).
Patil, S.T. et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 13, 1102–1107 (2007).
González-Maeso, J. et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97 (2008).
Agid, O., Kapur, S., Arenovich, T. & Zipursky, R.B. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch. Gen. Psychiatry 60, 1228–1235 (2003).
Egan, M.F. et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc. Natl. Acad. Sci. USA 101, 12604–12609 (2004).
Liu, W. et al. Pharmacogenetic analysis of the mGlu2/3 agonist LY2140023 monohydrate in the treatment of schizophrenia. Pharmacogenomics J. 12, 246–254 (2012).
Moreno, J.L. et al. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J. Neurosci. 31, 1863–1872 (2011).
Abdolmaleky, H.M. et al. Epigenetic dysregulation of HTR2A in the brain of patients with schizophrenia and bipolar disorder. Schizophr. Res. 129, 183–190 (2011).
Matrisciano, F., Tueting, P., Maccari, S., Nicoletti, F. & Guidotti, A. Pharmacological activation of group-II metabotropic glutamate receptors corrects a schizophrenia-like phenotype induced by prenatal stress in mice. Neuropsychopharmacology 37, 929–938 (2012).
Huang, H.S., Matevossian, A., Jiang, Y. & Akbarian, S. Chromatin immunoprecipitation in postmortem brain. J. Neurosci. Methods 156, 284–292 (2006).
Darcy, M.J., Calvin, K., Cavnar, K. & Ouimet, C.C. Regional and subcellular distribution of HDAC4 in mouse brain. J. Comp. Neurol. 518, 722–740 (2010).
González-Maeso, J. et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J. Neurosci. 23, 8836–8843 (2003).
Tamaru, Y., Nomura, S., Mizuno, N. & Shigemoto, R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience 106, 481–503 (2001).
Benneyworth, M.A. et al. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 72, 477–484 (2007).
Aghajanian, G.K. Modeling “psychosis” in vitro by inducing disordered neuronal network activity in cortical brain slices. Psychopharmacology (Berl.) 206, 575–585 (2009).
Zhai, Y. et al. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology 28, 45–52 (2003).
Kristiansen, L.V., Huerta, I., Beneyto, M. & Meador-Woodruff, J.H. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 7, 48–55 (2007).
Nestler, E.J. & Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169 (2010).
Geyer, M.A. & Vollenweider, F.X. Serotonin research: contributions to understanding psychoses. Trends Pharmacol. Sci. 29, 445–453 (2008).
Ludewig, K., Geyer, M.A. & Vollenweider, F.X. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol. Psychiatry 54, 121–128 (2003).
Chiechio, S. et al. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol. Pharmacol. 75, 1014–1020 (2009).
Tsankova, N., Renthal, W., Kumar, A. & Nestler, E.J. Epigenetic regulation in psychiatric disorders. Nat. Rev. Neurosci. 8, 355–367 (2007).
Guan, J.S. et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459, 55–60 (2009).
Gräff, J. et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483, 222–226 (2012).
Peter, C.J. & Akbarian, S. Balancing histone methylation activities in psychiatric disorders. Trends Mol. Med. 17, 372–379 (2011).
Marder, E. & Taylor, A.L. Multiple models to capture the variability in biological neurons and networks. Nat. Neurosci. 14, 133–138 (2011).
Dean, B. The cortical serotonin2A receptor and the pathology of schizophrenia: a likely accomplice. J. Neurochem. 85, 1–13 (2003).
Goldberg, T.E. et al. The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. Br. J. Psychiatry 162, 43–48 (1993).
Tsankova, N.M. et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 9, 519–525 (2006).
Humphrey, G.W. et al. Complementary roles for histone deacetylases 1, 2, and 3 in differentiation of pluripotent stem cells. Differentiation 76, 348–356 (2008).
Deacon, R.M. & Rawlins, J.N. T-maze alternation in the rodent. Nat. Protoc. 1, 7–12 (2006).
Hof, P.R., Young, W.G., Bloom, F.E., Belichenko, P.V. & Celio, M.R. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/Sv Mouse Brains (Elsevier, Amsterdam, 2000).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV 4th edn. (Washington, DC, 1994).
Stan, A.D. et al. Human postmortem tissue: what quality markers matter? Brain Res. 1123, 1–11 (2006).
Cao, J.L. et al. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci. 30, 16453–16458 (2010).
Martin, W.R., Wikler, A., Eades, C.G. & Pescor, F.T. Tolerance to and physical dependence on morphine in rats. Psychopharmacologia 4, 247–260 (1963).
González-Maeso, J., Rodriguez-Puertas, R., Meana, J.J., Garcia-Sevilla, J.A. & Guimon, J. Neurotransmitter receptor-mediated activation of G-proteins in brains of suicide victims with mood disorders: selective supersensitivity of alpha(2A)-adrenoceptors. Mol. Psychiatry 7, 755–767 (2002).
Shaffer, J.P. Multiple hypothesis testing. Annu. Rev. Psychol. 46, 561–584 (1995).
Acknowledgements
The authors would like to thank A. Chess, Y. Hurd, C. Alberini, P. Bos and J. Greally for critical review of the manuscript; M. Shapiro for help in working memory experiments; S. Morgello (Mount Sinai School of Medicine) and the Manhattan HIV Brain Bank for providing control brain cortex; P. Casaccia (Mount Sinai School of Medicine) for discussions and for providing the plasmid encoding mouse HDAC2; J. Gingrich (Columbia University) for his gift of 5HT2A−/− mice; V. Rayannavar for assistance with biochemical and behavioral assays; and the staff members of the Basque Institute of Legal Medicine for their cooperation in the study. US National Institutes of Health (NIH) R01 MH084894 (J.G.-M.), Dainippon Sumitomo Pharma (J.G.-M.), NARSAD (J.G.-M.), the Maltz Family Foundation (J.G.-M.), NIH R01 MH090264 (S.J.R.), MICINN SAF2009-084609 (J.J.M.), the Basque Government IT-199-07 (J.J.M.), NIH P50 MH090963 (E.J.N.), NIH R01 MH092306 (M.-H.H.) and NIH P50 MH066392 (J.D.B.) helped fund this study. A.K.F. was supported by NIH grant F32 MH096464. A.G.-B., J.L.M. and G.M. were recipients of predoctoral and postdoctoral fellowships from the Basque government, Ministerio de Ciencia e Innovación, and Consejo Superior de Investigaciones Científicas (CSIC), Spain.
Author information
Authors and Affiliations
Contributions
M.K. and J.G.-M. designed experiments, analyzed data and wrote the manuscript. M.K. performed experiments. J.G.-M. supervised the research. T.H. performed behavior experiments. A.G.-B. performed experiments in post-mortem schizophrenia-affected brain. A.K. assisted with cloning and performed promoter assay experiments. J.L.M. performed radioligand binding experiments. M.H. assisted with ChIP and DNA methylation assays. G.M., A.M.G., J.H., A.U. and A.L.G. assisted with experiments. R.L.N. performed viral packaging. L.S. performed biostatistical analyses. L.F.C. and J.J.M. obtained and classified post-mortem human brain samples. S.A.G., supervised by S.J.R., helped with stereotaxic surgery. P.J.K. and D.M.D., supervised by E.J.N., helped with viral overexpression. A.K.F., supervised by M.-H.H., performed electrophysiological studies. N.T., supervised by J.D.B., helped with prepulse inhibition test. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Tables 1–2 (PDF 4358 kb)
Rights and permissions
About this article
Cite this article
Kurita, M., Holloway, T., García-Bea, A. et al. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci 15, 1245–1254 (2012). https://doi.org/10.1038/nn.3181
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3181
This article is cited by
-
The neuropsychopharmacology of acetyl-L-carnitine (LAC): basic, translational and therapeutic implications
Discover Mental Health (2024)
-
Mechanisms and molecular targets surrounding the potential therapeutic effects of psychedelics
Molecular Psychiatry (2023)
-
Integrative omics of schizophrenia: from genetic determinants to clinical classification and risk prediction
Molecular Psychiatry (2022)
-
Sex-specific effects of psychedelics on prepulse inhibition of startle in 129S6/SvEv mice
Psychopharmacology (2022)
-
Effect of 5-HT2A receptor antagonism on levels of D2/3 receptor occupancy and adverse behavioral side-effects induced by haloperidol: a SPECT imaging study in the rat
Translational Psychiatry (2021)