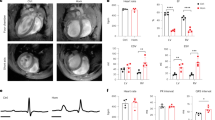
Abstract
Cardiac failure is the most common cause of mortality in Friedreich's ataxia (FRDA), a mitochondrial disease characterized by neurodegeneration, hypertrophic cardiomyopathy and diabetes1,2,3. FRDA is caused by reduced levels of frataxin (FXN), an essential mitochondrial protein involved in the biosynthesis of iron-sulfur (Fe-S) clusters4,5,6,7,8. Impaired mitochondrial oxidative phosphorylation, bioenergetics imbalance, deficit of Fe-S cluster enzymes and mitochondrial iron overload occur in the myocardium of individuals with FRDA9,10,11,12. No treatment exists as yet for FRDA cardiomyopathy13,14. A conditional mouse model with complete frataxin deletion in cardiac and skeletal muscle (Mck-Cre-FxnL3/L– mice) recapitulates most features of FRDA cardiomyopathy, albeit with a more rapid and severe course15,16. Here we show that adeno-associated virus rh10 vector expressing human FXN injected intravenously in these mice fully prevented the onset of cardiac disease. Moreover, later administration of the frataxin-expressing vector, after the onset of heart failure, was able to completely reverse the cardiomyopathy of these mice at the functional, cellular and molecular levels within a few days. Our results demonstrate that cardiomyocytes with severe energy failure and ultrastructure disorganization can be rapidly rescued and remodeled by gene therapy and establish the preclinical proof of concept for the potential of gene therapy in treating FRDA cardiomyopathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Harding, A.E. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 104, 589–620 (1981).
Tsou, A.Y. et al. Mortality in Friedreich ataxia. J. Neurol. Sci. 307, 46–49 (2011).
Weidemann, F. et al. The heart in Friedreich ataxia: definition of cardiomyopathy, disease severity, and correlation with neurological symptoms. Circulation 125, 1626–1634 (2012).
Campuzano, V. et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum. Mol. Genet. 6, 1771–1780 (1997).
Campuzano, V. et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 (1996).
Tsai, C.L. & Barondeau, D.P. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry 49, 9132–9139 (2010).
Schmucker, S. et al. Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS ONE 6, e16199 (2011).
Colin, F. et al. Mammalian frataxin controls sulfur production and iron entry during de novo Fe4S4 cluster assembly. J. Am. Chem. Soc. 135, 733–740 (2013).
Lodi, R. et al. Cardiac energetics are abnormal in Friedreich ataxia patients in the absence of cardiac dysfunction and hypertrophy: an in vivo31P magnetic resonance spectroscopy study. Cardiovasc. Res. 52, 111–119 (2001).
Lamarche, J., Shapcott, D., Côté, M. & Lemieux, B. Cardiac iron deposits in Friedreich's ataxia. in Handbook of Cerebellar Diseases (ed. Lechtenberg, R.) 453–457 (CRC Press, 1993).
Rötig, A. et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 17, 215–217 (1997).
Michael, S. et al. Iron and iron-responsive proteins in the cardiomyopathy of Friedreich's ataxia. Cerebellum 5, 257–267 (2006).
Perlman, S.L. A review of Friedreich ataxia clinical trial results. J. Child Neurol. 27, 1217–1222 (2012).
Wilson, R.B. Therapeutic developments in Friedreich ataxia. J. Child Neurol. 27, 1212–1216 (2012).
Puccio, H. et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 27, 181–186 (2001).
Seznec, H. et al. Idebenone delays the onset of cardiac functional alteration without correction of Fe-S enzymes deficit in a mouse model for Friedreich ataxia. Hum. Mol. Genet. 13, 1017–1024 (2004).
Hu, C., Busuttil, R.W. & Lipshutz, G.S. RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J. Gene Med. 12, 766–778 (2010).
Wang, G. et al. Persistent expression of biologically active anti-HER2 antibody by AAVrh.10-mediated gene transfer. Cancer Gene Ther. 17, 559–570 (2010).
Bernardo, B.C., Weeks, K.L., Pretorius, L. & McMullen, J.R. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol. Ther. 128, 191–227 (2010).
Wilkins, B.J. & Molkentin, J.D. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem. Biophys. Res. Commun. 322, 1178–1191 (2004).
Elia, L. et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120, 2377–2385 (2009).
Barry, W.H. & Bridge, J.H. Intracellular calcium homeostasis in cardiac myocytes. Circulation 87, 1806–1815 (1993).
Schmucker, S., Argentini, M., Carelle-Calmels, N., Martelli, A. & Puccio, H. The in vivo mitochondrial two-step maturation of human frataxin. Hum. Mol. Genet. 17, 3521–3531 (2008).
Guillon, B. et al. Frataxin deficiency causes upregulation of mitochondrial Lon and ClpP proteases and severe loss of mitochondrial Fe-S proteins. FEBS J. 276, 1036–1047 (2009).
Navarro-Sastre, A. et al. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am. J. Hum. Genet. 89, 656–667 (2011).
Huang, M.L. et al. Molecular and functional alterations in a mouse cardiac model of Friedreich ataxia: activation of the integrated stress response, eIF2α phosphorylation, and the induction of downstream targets. Am. J. Pathol. 183, 745–757 (2013).
Seznec, H. et al. Friedreich ataxia: the oxidative stress paradox. Hum. Mol. Genet. 14, 463–474 (2005).
Vorgerd, M. et al. Mitochondrial impairment of human muscle in Friedreich ataxia in vivo. Neuromuscul. Disord. 10, 430–435 (2000).
St John Sutton, M. et al. Longitudinal strain in friedreich ataxia: a potential marker for early left ventricular dysfunction. Echocardiography 31, 50–57 (2014).
Dedobbeleer, C., Rai, M., Donal, E., Pandolfo, M. & Unger, P. Normal left ventricular ejection fraction and mass but subclinical myocardial dysfunction in patients with Friedreich's ataxia. Eur. Heart J. Cardiovasc. Imaging 13, 346–352 (2012).
Raman, S.V. et al. Impaired myocardial perfusion reserve and fibrosis in Friedreich ataxia: a mitochondrial cardiomyopathy with metabolic syndrome. Eur. Heart J. 32, 561–567 (2011).
Wagner, G.R., Pride, P.M., Babbey, C.M. & Payne, R.M. Friedreich's ataxia reveals a mechanism for coordinate regulation of oxidative metabolism via feedback inhibition of the SIRT3 deacetylase. Hum. Mol. Genet. 21, 2688–2697 (2012).
Chan, D.C. Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 46, 265–287 (2012).
Sedlak, T.L., Chandavimol, M. & Straatman, L. Cardiac transplantation: a temporary solution for Friedreich's ataxia–induced dilated cardiomyopathy. J. Heart Lung Transplant. 23, 1304–1306 (2004).
Sondhi, D. et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol. Ther. 15, 481–491 (2007).
Rabinowitz, J.E. et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 76, 791–801 (2002).
Martelli, A. et al. Clinical data and characterization of the liver conditional mouse model exclude neoplasia as a non-neurological manifestation associated with Friedreich's ataxia. Dis. Model. Mech. 5, 860–869 (2012).
Acknowledgements
We thank A. Martelli for help in vector construction and for fruitful discussions, P. Bougnères for fruitful discussions and insightful comments on the manuscript, and V. Blouin and P. Moullier (Institut de Recherche Thérapeutique, INSERM UMR 1089) for vector production. This work was supported by the Association Française contre les Myopathies (to H.P.), the US Friedreich Ataxia Research Alliance (to H.P.), the European Community under the European Research Council (206634/ISCATAXIA; to H.P.), the Seventh Framework Programme (242193/EFACTS; to H.P.), the Association the Fondation Simone et Cino del Duca (to P.A.) and the Institut de France (to P.A.), by a personal donation from the Ledru family (to H.P.) and by a French state fund through the Agence Nationale de la Recherche under the frame programme Investissements d'Avenir labeled ANR-10-IDEX-0002-02 (ANR-10-LABX-0030-INRT). M.P. is a recipient of a PhD fellowship from the Association Française pour l'Ataxie de Friedreich.
Author information
Authors and Affiliations
Contributions
M.P. injected the mice, analyzed the echocardiographic results, and performed and analyzed the molecular and biochemical experiments; B.B. performed the survival, echocardiography and histological experiments; N.M. prepared and analyzed the samples for the electron microscopy analysis; L.M. trained B.B. for echocardiography and cardiac function analysis; L.R. was responsible for mouse production; R.G.C. and N.C. provided advice for the design of the study; M.P., B.B., P.A. and H.P. designed the study; H.P. and P.A. conceived of the study and were responsible for research coordination and strategy. M.P., B.B., P.A. and H.P. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
R.G.C., P.A. and H.P. are scientific founders of AAVLIFE, a gene therapy company focusing on rare diseases.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–8 (PDF 9619 kb)
Supplementary Data Sets
Source Data for Supplementary Figures and Tables (XLSX 47 kb)
Rights and permissions
About this article
Cite this article
Perdomini, M., Belbellaa, B., Monassier, L. et al. Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia. Nat Med 20, 542–547 (2014). https://doi.org/10.1038/nm.3510
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3510
This article is cited by
-
Patient-derived iPSC models of Friedreich ataxia: a new frontier for understanding disease mechanisms and therapeutic application
Translational Neurodegeneration (2023)
-
Quantification of human mature frataxin protein expression in nonhuman primate hearts after gene therapy
Communications Biology (2023)
-
Progress in Clinical Gene Therapy for Cardiac Disorders
Molecular Diagnosis & Therapy (2023)
-
AAV-vector based gene therapy for mitochondrial disease: progress and future perspectives
Orphanet Journal of Rare Diseases (2022)
-
Recessive cerebellar and afferent ataxias — clinical challenges and future directions
Nature Reviews Neurology (2022)