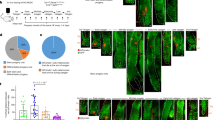
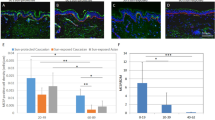
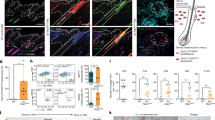
Abstract
During wound healing, stem cells provide functional mature cells to meet acute demands for tissue regeneration1. Simultaneously, the tissue must maintain a pool of stem cells to sustain its future regeneration capability. However, how these requirements are balanced in response to injury is unknown. Here we demonstrate that after wounding or ultraviolet type B irradiation, melanocyte stem cells (McSCs) in the hair follicle2 exit the stem cell niche before their initial cell division, potentially depleting the pool of these cells. We also found that McSCs migrate to the epidermis in a melanocortin 1 receptor (Mc1r)-dependent manner and differentiate into functional epidermal melanocytes, providing a pigmented protective barrier against ultraviolet irradiation over the damaged skin. These findings provide an example in which stem cell differentiation due to injury takes precedence over stem cell maintenance and show the potential for developing therapies for skin pigmentation disorders by manipulating McSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fuchs, E. Skin stem cells: rising to the surface. J. Cell Biol. 180, 273–284 (2008).
Nishimura, E.K. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–860 (2002).
Lin, J.Y. & Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 445, 843–850 (2007).
Hirobe, T. Histochemical survey of the distribution of the epidermal melanoblasts and melanocytes in the mouse during fetal and postnatal periods. Anat. Rec. 208, 589–594 (1984).
Nishimura, E.K. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 24, 401–410 (2011).
Cotsarelis, G., Sun, T.T. & Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Taylor, G., Lehrer, M.S., Jensen, P.J., Sun, T.T. & Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11, 1351–1354 (2005).
Ortonne, J.P., MacDonald, D.M., Micoud, A. & Thivolet, J. PUVA-induced repigmentation of vitiligo: a histochemical (split-DOPA) and ultrastructural study. Br. J. Dermatol. 101, 1–12 (1979).
Cui, J., Shen, L.Y. & Wang, G.C. Role of hair follicles in the repigmentation of vitiligo. J. Invest. Dermatol. 97, 410–416 (1991).
Zhao, S. & Overbeek, P.A. Tyrosinase-related protein 2 promoter targets transgene expression to ocular and neural crest-derived tissues. Dev. Biol. 216, 154–163 (1999).
Tanimura, S. et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 8, 177–187 (2011).
Rabanni, P. et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145, 941–955 (2011).
Lang, D. et al. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature 433, 884–887 (2005).
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10, 207–217 (2009).
Robbins, L.S. et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72, 827–834 (1993).
Hunt, G., Kyne, S., Wakamatsu, K., Ito, S. & Thody, A.J. Nle4DPhe7 α-melanocyte–stimulating hormone increases the eumelanin:phaeomelanin ratio in cultured human melanocytes. J. Invest. Dermatol. 104, 83–85 (1995).
Seong, I. et al. Sox10 controls migration of B16F10 melanoma cells through multiple regulatory target genes. PLoS ONE 7, e31477 (2012).
Ito, N. et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 19, 1332–1334 (2005).
Slominski, A., Wortsman, J., Luger, T., Paus, R. & Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 80, 979–1020 (2000).
Vukelic, S. et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J. Biol. Chem. 286, 10265–10275 (2011).
D'Orazio, J.A. et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature 443, 340–344 (2006).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Lichti, U. et al. In vivo regulation of murine hair growth: insights from grafting defined cell populations onto nude mice. J. Invest. Dermatol. 101 (suppl.), 124S–129S (1993).
Prouty, S.M., Lawrence, L. & Stenn, K.S. Fibroblast-dependent induction of a murine skin lesion with similarity to human common blue nevus. Am. J. Pathol. 148, 1871–1885 (1996).
Pharoah, P.D. Shedding light on skin cancer. Nat. Genet. 40, 817–818 (2008).
Gilchrest, B.A. Molecular aspects of tanning. J. Invest. Dermatol. 131, E14–E17 (2011).
Inoue, K. et al. Stress augmented ultraviolet-irradiation–induced pigmentation. J. Invest. Dermatol. 121, 165–171 (2003).
Bennett, D.C., Cooper, P.J. & Hart, I.R. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer 39, 414–418 (1987).
Han, R., Baden, H.P., Brissette, J.L. & Weiner, L. Redefining the skin's pigmentary system with a novel tyrosinase assay. Pigment Cell Res. 15, 290–297 (2002).
Acknowledgements
Follicle-derived epidermal melanocytes were initially observed at G. Cotsarelis' laboratory at the University of Pennsylvania, and this work benefited greatly from the mentorship and generosity of G. Cotsarelis. We thank P. Manga at New York University (NYU) for valuable discussion and for providing melan-a cells and antibody to mouse tyrosinase. We thank E. Hernando's lab at NYU for the protocol of the melanocyte migration assay. We thank the Microscopy Core of NYU for use of confocal microscopes (NCRRS10 RR023704-01A1). M.T. is supported by the NYU Kimmel Stem Cell Center and NYSTEM training grant C026880. M.I. is supported by US National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1R01AR059768-01A1 and the Ellison Medical Foundation.
Author information
Authors and Affiliations
Contributions
W.C.C. performed experiments, interpreted data and wrote the manuscript. M.T., P.R., H.H., W.L. and Y.R.C. performed experiments and interpreted data. J.C. provided human skin and interpreted data. P.O. generated Trp2-lacZ mice and interpreted the data. M.I. performed experiments, interpreted data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1059 kb)
Rights and permissions
About this article
Cite this article
Chou, W., Takeo, M., Rabbani, P. et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med 19, 924–929 (2013). https://doi.org/10.1038/nm.3194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3194
This article is cited by
-
Reproducible strategy for excisional skin-wound-healing studies in mice
Nature Protocols (2024)
-
Sexual dimorphism in melanocyte stem cell behavior reveals combinational therapeutic strategies for cutaneous repigmentation
Nature Communications (2024)
-
Deciphering the molecular mechanisms of stem cell dynamics in hair follicle regeneration
Experimental & Molecular Medicine (2024)
-
Dedifferentiation maintains melanocyte stem cells in a dynamic niche
Nature (2023)
-
The journey from melanocytes to melanoma
Nature Reviews Cancer (2023)