Abstract
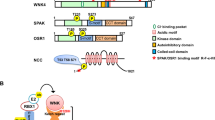
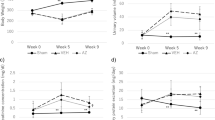
Calcineurin inhibitors (CNIs) are immunosuppressive drugs that are used widely to prevent rejection of transplanted organs and to treat autoimmune disease. Hypertension and renal tubule dysfunction, including hyperkalemia, hypercalciuria and acidosis, often complicate their use1,2. These side effects resemble familial hyperkalemic hypertension, a genetic disease characterized by overactivity of the renal sodium chloride cotransporter (NCC) and caused by mutations in genes encoding WNK kinases. We hypothesized that CNIs induce hypertension by stimulating NCC. In wild-type mice, the CNI tacrolimus caused salt-sensitive hypertension and increased the abundance of phosphorylated NCC and the NCC-regulatory kinases WNK3, WNK4 and SPAK. We demonstrated the functional importance of NCC in this response by showing that tacrolimus did not affect blood pressure in NCC-knockout mice, whereas the hypertensive response to tacrolimus was exaggerated in mice overexpressing NCC. Moreover, hydrochlorothiazide, an NCC-blocking drug, reversed tacrolimus-induced hypertension. These observations were extended to humans by showing that kidney transplant recipients treated with tacrolimus had a greater fractional chloride excretion in response to bendroflumethiazide, another NCC-blocking drug, than individuals not treated with tacrolimus; renal NCC abundance was also greater. Together, these findings indicate that tacrolimus-induced chronic hypertension is mediated largely by NCC activation, and suggest that inexpensive and well-tolerated thiazide diuretics may be especially effective in preventing the complications of CNI treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
13 January 2012
In the version of this article initially published, the dose and route of administration of tacrolimus were inadvertently omitted. Tacrolimus or vehicle was administered subcutaneously; the dose of tacrolimus was 1 mg per kg body weight per day. The error has been corrected in the HTML and PDF versions of the article.
References
Kim, H.C. et al. Primary immunosuppression with tacrolimus in kidney transplantation: three-year follow-up in a single center. Transplant. Proc. 36, 2082–2083 (2004).
Jain, A. et al. What have we learned about primary liver transplantation under tacrolimus immunosuppression? Long-term follow-up of the first 1000 patients. Ann. Surg. 230, 441–448, discussion 448–449 (1999).
Nijenhuis, T., Hoenderop, J.G. & Bindels, R.J. Downregulation of Ca2+ and Mg2+ transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J. Am. Soc. Nephrol. 15, 549–557 (2004).
Mohebbi, N., Mihailova, M. & Wagner, C.A. The calcineurin inhibitor FK506 (tacrolimus) is associated with transient metabolic acidosis and altered expression of renal acid-base transport proteins. Am. J. Physiol. Renal Physiol. 297, F499–F509 (2009).
Takeda, Y., Miyamori, I., Furukawa, K., Inaba, S. & Mabuchi, H. Mechanisms of FK 506-induced hypertension in the rat. Hypertension 33, 130–136 (1999).
Curtis, J.J., Luke, R.G., Jones, P. & Diethelm, A.G. Hypertension in cyclosporine-treated renal transplant recipients is sodium dependent. Am. J. Med. 85, 134–138 (1988).
Kang, C.B., Hong, Y., Dhe-Paganon, S. & Yoon, H.S. FKBP family proteins: immunophilins with versatile biological functions. Neurosignals 16, 318–325 (2008).
Smith, K.D. et al. Delayed graft function and cast nephropathy associated with tacrolimus plus rapamycin use. J. Am. Soc. Nephrol. 14, 1037–1045 (2003).
Gooch, J.L., Roberts, B.R., Cobbs, S.L. & Tumlin, J.A. Loss of the alpha-isoform of calcineurin is sufficient to induce nephrotoxicity and altered expression of transforming growth factor-beta. Transplantation 83, 439–447 (2007).
McCormick, J.A., Nelson, J.H., Yang, C.L., Curry, J.N. & Ellison, D.H. Overexpression of the sodium chloride cotransporter is not sufficient to cause familial hyperkalemic Hypertension. Hypertension published online, doi:10.1161HYPERTENSIONAHA.110.167809 (6 September 2011).
Esteva-Font, C. et al. Ciclosporin-induced hypertension is associated with increased sodium transporter of the loop of Henle (NKCC2). Nephrol. Dial. Transplant. 22, 2810–2816 (2007).
Anselmo, A.N. et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc. Natl. Acad. Sci. USA 103, 10883–10888 (2006).
Yang, C.L., Angell, J., Mitchell, R. & Ellison, D.H. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J. Clin. Invest. 111, 1039–1045 (2003).
San-Cristobal, P. et al. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc. Natl. Acad. Sci. USA 106, 4384–4389 (2009).
Melnikov, S., Mayan, H., Uchida, S., Holtzman, E.J. & Farfel, Z. Cyclosporine metabolic side effects: association with the WNK4 system. Eur. J. Clin. Invest. 41, 1113–1120 (2011).
Schultheis, P.J. et al. Phenotype resembling Gitelman's syndrome in mice lacking the apical Na+-Cl− cotransporter of the distal convoluted tubule. J. Biol. Chem. 273, 29150–29155 (1998).
Hu, D.C., Burtner, C., Hong, A., Lobo, P.I. & Okusa, M.D. Correction of renal hypertension after kidney transplantation from a donor with Gitelman syndrome. Am. J. Med. Sci. 331, 105–109 (2006).
Colussi, G. et al. A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin. J. Am. Soc. Nephrol. 2, 454–460 (2007).
Madala Halagappa, V.K., Tiwari, S., Riazi, S., Hu, X. & Ecelbarger, C.M. Chronic candesartan alters expression and activity of NKCC2, NCC, and ENaC in the obese Zucker rat. Am. J. Physiol. 294, F1222–F1231 (2008).
Feng, M. et al. Genetic analysis of blood pressure in 8 mouse intercross populations. Hypertension 54, 802–809 (2009).
Koomans, H.A. & Ligtenberg, G. Mechanisms and consequences of arterial hypertension after renal transplantation. Transplantation 72, S9–S12 (2001).
Curtis, J.J. Hypertensinogenic mechanism of the calcineurin inhibitors. Curr. Hypertens. Rep. 4, 377–380 (2002).
Segal, A.S., Hayslett, J.P. & Desir, G.V. On the natriuretic effect of verapamil: inhibition of ENaC and transepithelial sodium transport. Am. J. Physiol. Renal Physiol. 283, F765–F770 (2002).
Paver, W.K. & Pauline, G.J. Hypertension and hyperpotassemia without renal disease in a young male. Med. J. Aust. 2, 305–306 (1964).
Calò, L., Davis, P.A. & Semplicini, A. Control of vascular tone in the syndromes of Bartter and Gitelman. Crit. Rev. Clin. Lab. Sci. 37, 503–522 (2000).
Calò, L., Davis, P.A. & Semplicini, A. Reduced content of alpha subunit of Gq protein content in monocytes of Bartter and Gitelman syndromes: relationship with vascular hyporeactivity. Kidney Int. 61, 353–354 (2002).
Guyton, A.C. Blood pressure control—special role of the kidneys and body fluids. Science 252, 1813–1816 (1991).
Adu, D., Michael, J., Turney, J. & McMaster, P. Hyperkalemia in cyclosporine treated renal allograft recipients. Lancet 322, 370–372 (1983).
Heering, P.J. et al. Aldosterone resistance in kidney transplantation is in part induced by a down-regulation of mineralocorticoid receptor expression. Clin. Transplant. 18, 186–192 (2004).
Higgins, R. et al. Hyponatraemia and hyperkalaemia are more frequent in renal transplant recipients treated with tacrolimus than with cyclosporin. Further evidence for differences between cyclosporin and tacrolimus nephrotoxicities. Nephrol. Dial. Transplant. 19, 444–450 (2004).
Van Laecke, S. et al. Posttransplantation hypomagnesemia and its relation with immunosuppression as predictors of new-onset diabetes after transplantation. Am. J. Transplant 9, 2140–2149 (2009).
Arthur, J.M. & Shamim, S. Interaction of cyclosporine and FK506 with diuretics in transplant patients. Kidney Int. 58, 325–330 (2000).
Schindler, R., Tanriver, Y. & Frei, U. Hypertension and allograft nephropathy—cause, consequence, or both? Nephrol. Dial. Transplant. 15, 8–10 (2000).
Feng, M. et al. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am. J. Hypertens. 21, 1288–1291 (2008).
Kurtz, T.W., Griffin, K.A., Bidani, A.K., Davisson, R.L. & Hall, J.E. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: Blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45, 299–310 (2005).
Yang, C.-L., Zhu, X. & Ellison, D.H. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J. Clin. Invest. 117, 3403–3411 (2007).
Welker, P. et al. Renal Na+-K+-Cl− cotransporter activity and vasopressin-induced trafficking are lipid raft-dependent. Am. J. Physiol. 295, F789–F802 (2008).
Piechotta, K., Lu, J. & Delpire, E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J. Biol. Chem. 277, 50812–50819 (2002).
Yang, S.S. et al. Mechanisms for hypercalciuria in pseudohypoaldosteronism type II–causing WNK4 knock-in mice. Endocrinology 151, 1829–1836 (2010).
Acknowledgements
For technical assistance, we thank N. Desmerais (animal studies), S. Rogers, K. Risowsky (immunohistochemistry), D. Gannon and A. Bakke (tacrolimus measurements in mice). We thank G. Jones and M. Harber for help in identifying human subjects. We also thank G. Shull (University of Cincinnati) for the NCC-knockout mice, and E. Delpire (Vanderbilt Kennedy Center) for SPAK antibodies. E.J.H. is supported by an Erasmus Medical Center Fellowship and Kolff Junior Postdoc Grant (Dutch Kidney Foundation, KJPB 08.004); J.A.M. is supported by the US National Institutes of Health (NIH) (K01 DK076617); D.H.E. is supported by the NIH (RO1 DK51496), the Department of Veterans Affairs (Merit Review) and the American Heart Association (10 GRNT 2630199). Portions of this work were presented at the High Blood Pressure Research Council Meeting of the American Heart Association, 13–16 October 2010, and the American Society of Nephrology, 15–18 November 2010.
Author information
Authors and Affiliations
Contributions
E.J.H. and S.B.W. carried out most of the experiments, analyzed the data and wrote the initial manuscript. J.A.M. generated the mice overexpressing the NCC and participated in animal experiments and analyses. J.C. did the aldosterone infusion experiments. A.F. contributed to the human experiments and, together with A.J.H., to the kidney biopsy tissue staining. C.-L.Y. conducted the cell studies. T.R., A.P. and S.B. carried out the calcineurin immunohistochemistry. R.J.U. and D.H.E. conceived of the study, supervised the work and edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–6 and Supplementary Figures 1–4 (PDF 379 kb)
Rights and permissions
About this article
Cite this article
Hoorn, E., Walsh, S., McCormick, J. et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat Med 17, 1304–1309 (2011). https://doi.org/10.1038/nm.2497
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2497
This article is cited by
-
Preclinical assessment of antigen-specific chimeric antigen receptor regulatory T cells for use in solid organ transplantation
Gene Therapy (2023)
-
Effects of pediatric chronic kidney disease and its etiology on tissue sodium concentration: a pilot study
Pediatric Nephrology (2023)
-
Role of hypertension in kidney transplant recipients
Journal of Human Hypertension (2021)
-
Inhibition of big-conductance Ca2+-activated K+ channels in cerebral artery (vascular) smooth muscle cells is a major novel mechanism for tacrolimus-induced hypertension
Pflügers Archiv - European Journal of Physiology (2021)
-
Mechanisms and management of drug-induced hyperkalemia in kidney transplant patients
Reviews in Endocrine and Metabolic Disorders (2021)