Abstract
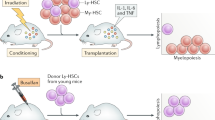
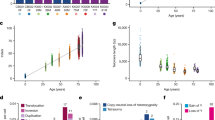
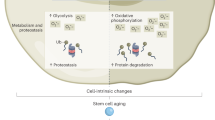
The extensive self-renewal capacity of hematopoietic stem cells (HSCs) implies that this cell population may not age and thus may provide undiminished replenishment of blood cells throughout the lifespan of an organism. In contrast, accumulating experimental evidence supports the premise that HSCs show signs of aging and may have a limited functional lifespan. We summarize here the evidence for HSC aging, discuss the possible molecular mechanisms that may be involved and show evidence of a genetic connection between the effects of age on blood-forming cells and the longevity of mice. We speculate that age-related functional decline in adult tissue HSCs limits longevity in mammals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Harrison, D. E. Normal function of transplanted mouse erythrocyte precursors for 21 months beyond donor life spans. Nature New Biology 237, 220–222 (1972).
Ploemacher, R. E. & Brons, N. H. C. Isolation of hemopoietic stem cell subsets from murine bone marrow: I. Radioprotective ability of purified cell suspensions differing in the proportion of day-7 and day-12 CFU-S. Exp. Hematol. 16, 21–26 (1988).
Spangrude, G. J., Heimfeld, S. & Weissman, I. L. Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 (1988).
Baum, C. M., Weissman, I. L., Tsukamoto, A. S., Buckle, A.-M. & Peault, B. Isolation of a candidate human hematopoietic stem-cell population. Proc. Natl Acad. Sci. USA 89, 2804–2808 (1992).
Morrison, S. & Weissman, I. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1, 661–673 (1994).
Zhao, Y. et al. Murine hematopoietic stem cell characterization and its regulation in BM transplantation. Blood 96, 3016–3022 (2000).
Morrison, S. J., Wandycz, A. M., Akashi, K., Globerson, A. & Weissman, I. L. The aging of hematopoietic stem cells. Nature Med. 2, 1011–1016 (1996).
Sudo, K., Ema, H., Morita, Y. & Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 192, 1273–1280 (2000).
de Haan, G. & Van Zant, G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood 93, 3294–3301 (1999).
Harrison, D., Astle, C. & Stone, M. Numbers and functions of transplantable primitive immunohemtopoietic stem cells. Effects of age. J. Immunol. 142, 3833–3840 (1989).
Ross, E., Anderson, N. & Micklem, H. S. Serial depletion and regeneration of the murine hematopoietic system. Implications for hematopoietic organization and the study of cellular aging. J. Exp. Med. 155, 432–444 (1982).
Harrison, D. Long-term erythropoietic repopulating ability of old, young and fetal stem cells. J. Exp. Med. 157, 1496–1504 (1983).
Rebel, V. I., Miller, C. L., Eaves, C. J. & Lansdorp, P. M. The repopulation potential of fetal liver hematopoietic stem cells in mice exceeds that of their adult bone marrow counterparts. Blood 87, 3500–3507 (1996).
Micklem, H. S., Ford, C. E., Evans, E. P., Ogden, D. A. & Papworth, D. S. Competitive in vivo proliferation of foetal and adult hematopoietic cells in lethally irradiated mice. J. Cell Physiol. 79, 293–298 (1972).
Harrison, D. E., Zhong, R. K., Jordan, C. T., Lemischka, I. R. & Astle, C. M. Relative to adult marrow, fetal liver repopulates nearly five times more effectively long-term than short-term. Exp. Hematol. 25, 293–297 (1997).
Chen, J., Astle, B. A. & Harrison, D. E. Development and aging of primitive hematopoietic stem cells in BALB/cBy mice. Exp. Hematol. 27, 928–935 (1999).
Chen, J., Astle, C. M. & Harrison, D. E. Genetic regulation of primitive hematopoietic stem cell senescence. Exp. Hematol. 28, 442–50 (2000).
Lansdorp, P., Dragowska, W. & Manyani, H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J. Exp. Med. 178, 787–791 (1993).
Van Zant, G., Holland, B. P., Eldridge, P. W. & Chen, J.-J. Genotype-restricted growth and aging patterns in hematopoietic stem cell populations of allophenic mice. J. Exp. Med. 171, 1547–1565 (1990).
Mintz, B. & Silvers, W. K. “Intrinsic” immunological tolerance in allophenic mice. Science 158, 1484–1486 (1967).
Van Zant, G., Scott-Micus, K., Thompson, B. P., Fleischman, R. A. & Perkins, S. Stem cell quiescence/activation is reversible by serial transplantation and is independent of stromal cell genotype in mouse aggregation chimeras. Exp. Hematol. 20, 470–475 (1992).
Campisi, J. Replicative senescence: An old lives' tale? Cell 84, 497–500 (1996).
Campisi, J. The biology of replicative senescence. Eur. J. Cancer 33, 703–709 (1997).
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P. Y. & Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl Acad. Sci. USA 98, 12072–12077 (2001).
de Haan, G. et al. Distinct functional properties of highly purified hematopoietic stem cells from mouse strains differing in stem cell numbers. Blood 96, 1374–1379 (2000).
Wineman, J., Moore, K., Lemischka, I. & Muller-Sieburg, C. Functional heterogeneity of the hematopoietic microenvironment: rare stromal elements maintain long-term repopulating stem cells. Blood 87, 4082–4090 (1996).
Cashman, J., Eaves, A. C. & Eaves, C. J. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood 66, 1002–1005 (1985).
Mauch, P., Botnick, L. E., Hannon, E. C., Obbagy, J. & Hellman, S. Decline in bone marrow proliferative capacity as a function of age. Blood 60, 245–252 (1982).
Chertkov, J. L. & Gurevitch, O. A. Age-related changes in hemopoietic microenvironment. Enhanced growth of hemopoietic stroma and weakened genetic resistance of hemopoietic cells in old mice. Exp. Gerontol. 16, 195–198 (1981).
Hotta, T., Hirabayashi, N., Utsumi, M., Murate, T. & Yamada, H. Age related changes in the function of hemopoietic stroma in mice. Exp. Hematol. 8, 933–936 (1980).
Jiang, D., Fei, R.-G., Pendergrass, W. & Wolf, N. An age-related reduction in the replicative capacity of two murine hematopoietic stroma cell types. Exp. Hematol. 20, 1216–1222 (1992).
Aspinall, R. & Andrew, D. Thymic involution in aging. J. Clin. Immunol. 20, 250–256 (2000).
Globerson, A. Thymocytopoiesis in aging: The bone marrow thymus axis. Arch. Gerontol. Geriatr. 24, 141–155 (1997).
Aspinall, R. Longevity and the immune response. Biogerontology 1, 273–278 (2000).
Aspinall, R. & Andrew, D. Age-associated thymic atrophy is not associated with a deficiency in the CD44+CD25−CD3−CD4−CD8− thymocyte population. Cell. Immunol. 212, 150–157 (2001).
Andrew, D. & Aspinall, R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp. Gerontol. 37, 455–463 (2002).
Bhatia, S. K., Tygrett, L. T., Grabstein, K. H. & Waldschmidt, T. J. The effect of in vivo IL-7 deprivation on T cell maturation. J. Exp. Med. 181, 1399–1409 (1995).
Miller, R. A. Effect of aging on T lymphocyte activation. Vaccine 18, 1654–1660 (2000).
Miller, R. A. The aging immune system: primer and prospectus. Science 273, 70–74 (1996).
Effros, R. B. Replicative senescence in the immune system: impact of the Hayflick limit on T-cell function in the elderly. Am. J. Hum. Genet. 62, 1003–1007 (1998).
Hsu, H. C. et al. Aged mice exhibit in vivo defective peripheral clonal deletion of D(b)/H-Y reactive CD8(+) T cells. Mech. Ageing Dev. 122, 305–326 (2001).
de Haan, G. & Van Zant, G. Genetic analysis of hemopoietic cell cycling in mice suggests its involvement in organismal life span. FASEB J. 13, 707–713 (1999).
Geiger, H., True, J. M., de Haan, G. & Van Zant, G. Age- and stage-specific regulation patterns in the hematopoietic stem cell hierarchy. Blood 98, 2966–2972 (2001).
de Haan, G. & Van Zant, G. Intrinsic and extrinsic control of hemopoietic stem cell numbers: Mapping of a stem cell gene. J. Exp. Med. 186, 529–536 (1997).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. J. Exp. Cell Res. 25, 585–621 (1961).
Hayflick, L. How and why we age. Exp. Gerontol. 33, 639–653 (1997).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Vaziri, H. et al. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc. Natl Acad. Sci. USA. 91, 9857–9860 (1994).
Hemann, M. T. & Greider, C. W. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 28, 4474–4478 (2000).
Morrison, S. J., Prowse, K. R., Ho, P. & Weissman, I. L. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity 5, 207–216 (1996).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Kennedy, B. K. et al. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell 89, 381–391 (1997).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Wright, W. E. & Shay, J. W. Cellular senescence as a tumor-protection mechanism: the essential role of counting. Curr. Opin. Genet. Dev. 11, 98–103 (2001).
Lundberg, A. S., Hahn, W. C., Gupta, P. & Weinberg, R. A. Genes involved in senescence and immortalization. Curr. Opin. Cell. Biol. 12, 705–709 (2000).
Kruk, P. A., Rampino, N. J. & Bohr, V. A. DNA damage and repair in telomeres: relation to aging. Proc. Natl Acad. Sci. USA 92, 258–62 (1995).
Imai, T., Jiang, M., Kastner, P., Chambon, P. & Metzger, D. Selective ablation of retinoid X receptor α in hepatocytes impairs their lifespan and regenerative capacity. Proc. Natl Acad. Sci. USA 98, 4581–6 (2001).
Dolle, M. et al. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nature Genet. 17, 431–434 (1997).
Dolle, M. E., Snyder, W. K., Gossen, J. A., Lohman, P. H. M. & Vijg, J. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc. Natl Acad. Sci. USA 97, 8403–8408 (2000).
Wang, Y. et al. Muscle-specific mutations accumulate with aging in critical human mtDNA control sites for replication. Proc. Natl Acad. Sci. USA 98, 4022–4027 (2001).
Hamilton, M. L. et al. Does oxidative damage to DNA increase with age? Proc. Natl Acad. Sci. USA 98, 10469–10474 (2001).
Hasty, P. The impact energy metabolism and genome maintenance have on longevity and senescence: lessons from yeast to mammals. Mech. Ageing Dev. 122, 1651–1662 (2001).
Bohr, V., Anson, R. M., Mazur, S. & Dianov, G. Oxidative DNA damage processing and changes with aging. Toxicol. Lett. 103, 47–52 (1998).
Anson, R. M., Hudson, E. & Bohr, V. A. Mitochondrial endogenous oxidative damage has been overestimated. FASEB J. 14, 355–360 (2000).
Jacobson, L. O., Marks, E. K., Robson, M. J., Gaston, E. O. & Zirkle, R. E. Effect of spleen protection on mortality following x-irradiation. J. Lab. Clin. Med. 34, 1538–1543 (1949).
Acknowledgements
Supported by the Bundesministerium für Bildung und Forschung by Leopoldina (H. G.) and NIH grant AG16653.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geiger, H., Van Zant, G. The aging of lympho-hematopoietic stem cells. Nat Immunol 3, 329–333 (2002). https://doi.org/10.1038/ni0402-329
Issue Date:
DOI: https://doi.org/10.1038/ni0402-329
This article is cited by
-
Analysis of the dynamic changes in the proportion of immune cells and the proportion of cells with stem cell characteristics in the corresponding immune cell population of C57 mice during the natural aging process
Immunologic Research (2021)
-
Smoking and Physical Activity Significantly Influence Stromal Vascular Fraction Cell Yield and Viability
Aesthetic Plastic Surgery (2021)